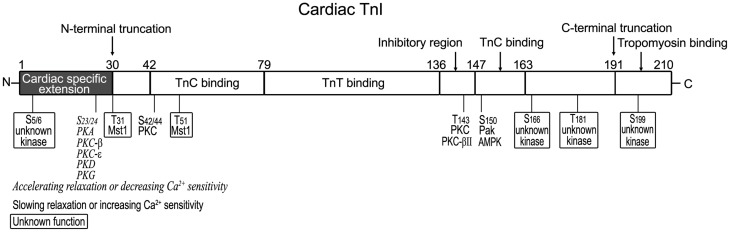
Figure 2.
Structural and functional domains of cardiac TnI and posttranslational modifications. Indicated on this linear map of cardiac TnI polypeptide (residue # corresponds to that in human sequence including Met1), Ser23/Ser24 are phosphorylated by PKA (Solaro and Kobayashi, 2011), decreasing Ca2+ sensitivity and accelerating relaxation. They have also been reported to be phosphorylated by PKC-β, PKC-ε (Kobayashi et al., 2005), PKD (Haworth et al., 2004; Cuello et al., 2007; Bardswell et al., 2010) and PKG (Layland et al., 2002). Ser42/Ser44, Thr143, and Ser150 are phosphorylated by PKC, Pak or AMPK, decreasing Ca2+ sensitivity and slowing relaxation (Macgowan et al., 2001; Buscemi et al., 2002; Pi et al., 2002; Burkart et al., 2003b; Westfall et al., 2005). Thr31 and Thr51 are phosphorylated by Mst1 (You et al., 2009) with unknown function. New phosphorylation sites have been identified in the N-terminal region (Ser5/Ser6, with decreased levels in heart failure) and in the C-terminal region (Ser166/Thr181/Ser199, with unknown kinases and functions) (Zhang et al., 2012). A restrictive N-terminal truncation of cardiac TnI occurs in adaptation to hemodynamic stresses to selectively remove the adult heart-specific N-terminal extension with an effect on increasing myocardial relaxation, similar to the effect of PKA phosphorylation at Ser23/Ser24 (Barbato et al., 2005). A deletion of the C-terminal 19 amino acids was found in myocardial ischemia-reperfusion injury (McDonough et al., 1999) and myocardial stunning (McDonough et al., 2001), slowing down the rates of force development and relaxation (Narolska et al., 2006).