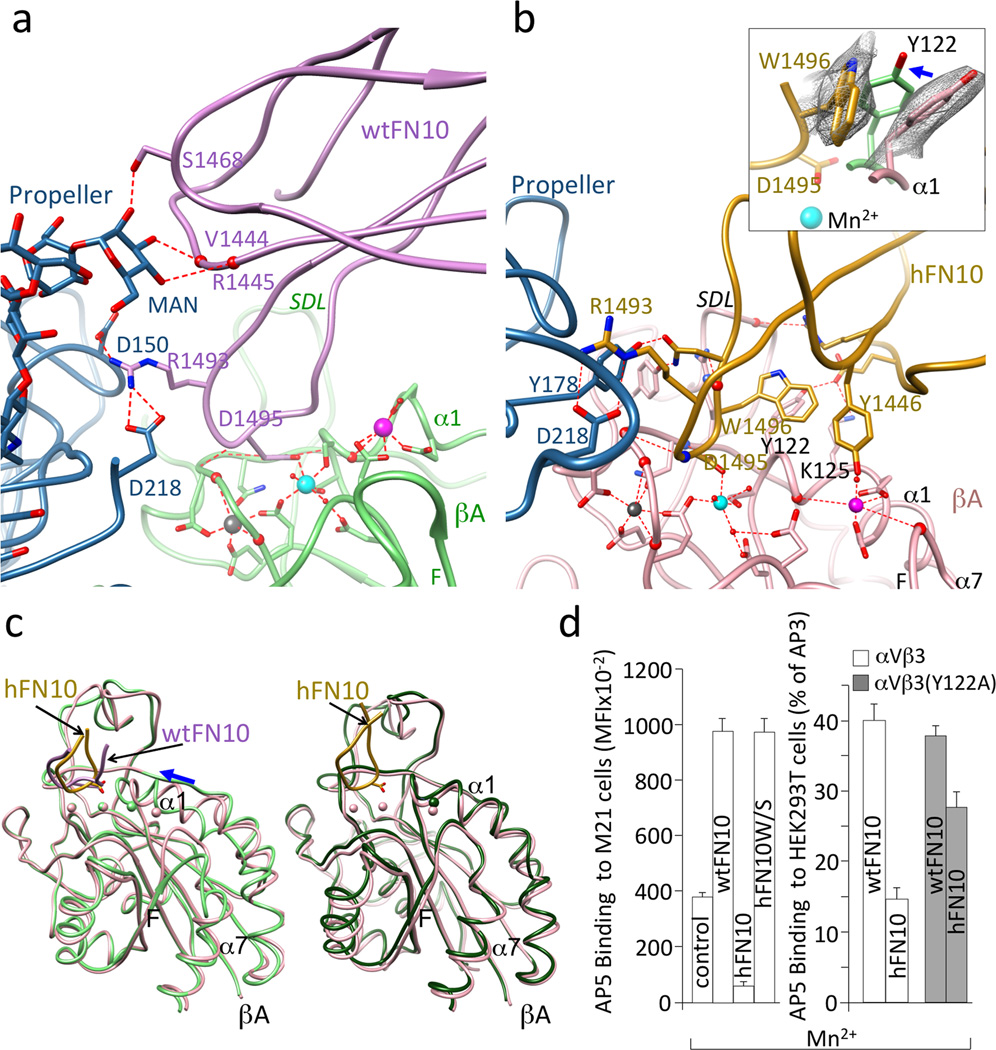
Figure 3. αVβ3-FN10 interfaces, conformational changes and structure validation.
Ribbon diagrams showing key electrostatic and H-bond interactions and metal ion coordinations in αVβ3-wtFN10 (a) and αVβ3-hFN10 (b) structures. Chain colors are as in Fig. 2. Inset in b, enlarged view of σA weighted 2Fo-Fc map contoured at 1.0σ of Trp1496 and Tyr122 side-chains in αVβ3-hFN10 complex. Inward movement (blue arrow) of Tyr122 (in light green) in wtFN10-bound βA would clash with Trp1496 side chain. (c) Left panel, βA domain from αVβ3-hFN10 (in pink) superimposed on that of αVβ3-wtFN10 (in light green) and on βA domain (in dark green) from unliganded αVβ3 (pdb 3ije) (right panel). Blue arrow in left panel in (c) indicates direction of wtFN10-induced inward movement of α1 helix (and ADMIDAS ion) towards MIDAS. Spheres representing the three metal ions bear the color of respective βA. The major tertiary change observed in the F-α7 loop of wtFN10-bound βA (c, left panel) was not translated into a one-turn displacement of α7, possibly the result of crystal contacts when the complete ectodomain is used in crystallization. Except for ligand-occupancy and resulting changes in SDL loop, structures of unliganded- and hFN10-bound βA domains are identical (c, right panel) (LIMBS and MIDAS are not occupied by metal in unliganded βA). (d) Left panel, binding of fluoresceinated AP5 to M21 cells in absence (control) or presence of unlabeled wtFN10, hFN10 or hFN10W/S. Right panel, binding of fluoresceinated AP5 to αVβ3+ or or αVβ3(Y122A) + HEK293T in presence of unlabeled wt- or hFN10. Histograms represent mean±SD, n=3 independent experiments.