Figure 2.
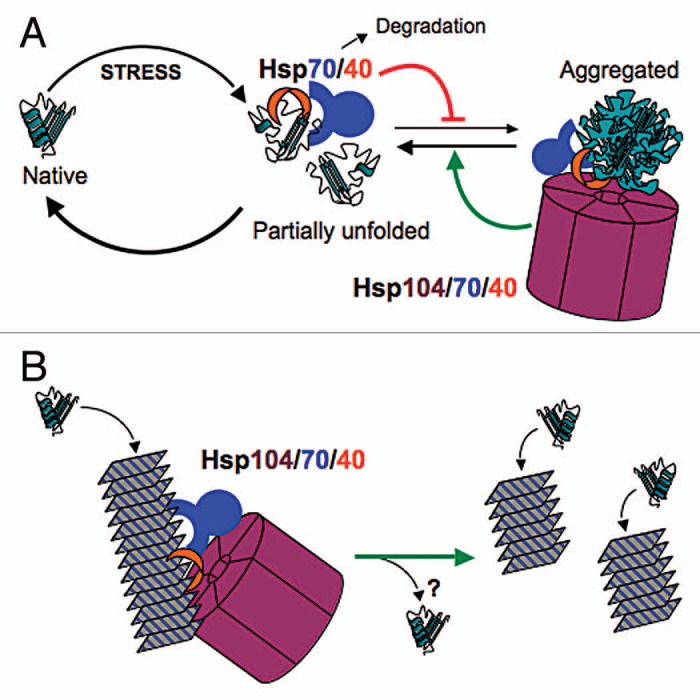
Models for the action of protein chaperones in stress protection (A) and yeast prion propagation (B). (A) Hsp70 (blue) and Hsp40 (red) prevent aggregation of proteins by binding and masking exposed hydrophobic surfaces on partially unfolded proteins. Release of the substrate, catalyzed by nucleotide exchange factors (not shown), provides an opportunity for the protein to regain its native conformation. Hsp70 can also promote degradation of misfolded proteins. Hsp104 (purple), assisted by Hsp70 and Hsp40, resolubilizes proteins from aggregates by extruding individual polypeptides through its axial pore. The combined action of Hsp70, Hsp40 and Hsp104 drives the reaction toward the native state. (B) A similar reaction of the Hsp104/70/40 machine as in (A) but acting on a prion polymer, which is shown as stacked rectangles representing amyloid. Extraction of a monomer from within the polymer by the Hsp104/70/40 system destabilizes the fiber, allowing it to break into pieces, each of which continues to propagate the amyloid structure. The fate of the extracted monomer is uncertain (question mark). In order for the prion to be transmitted efficiently to daughter cells, new fibers must be created continually by the action of this chaperone machinery.
