Abstract
Insulin-producing cells (IPCs) in the Drosophila brain produce and release insulin-like peptides (ILPs) to the hemolymph. ILPs are crucial for growth and regulation of metabolic activity in flies, functions analogous to those of mammalian insulin and insulin-like growth factors (IGFs). To identify components functioning in IPCs to control ILP production, we employed genomic and candidate gene approaches. We used laser microdissection and messenger RNA sequencing to characterize the transcriptome of larval IPCs. IPCs highly express many genes homologous to genes active in insulin-producing β-cells of the mammalian pancreas. The genes in common encode ILPs and proteins that control insulin metabolism, storage, secretion, β-cell proliferation, and some not previously linked to insulin production or β-cell function. Among these novelties is unc-104, a kinesin 3 family gene, which is more highly expressed in IPCs compared to most other neurons. Knockdown of unc-104 in IPCs impaired ILP secretion and reduced peripheral insulin signaling. Unc-104 appears to transport ILPs along axons. As a complementary approach, we tested dominant-negative Rab genes to find Rab proteins required in IPCs for ILP production or secretion. Rab1 was identified as crucial for ILP trafficking in IPCs. Inhibition of Rab1 in IPCs increased circulating sugar levels, delayed development, and lowered weight and body size. Immunofluorescence labeling of Rab1 showed its tight association with ILP2 in the Golgi of IPCs. Unc-104 and Rab1 join other proteins required for ILP transport in IPCs.
Keywords: insulin, pancreas, Drosophila, Unc-104, kinesin, Rab1, Golgi, ER, RNA-seq, laser microdissection, transport
SIGNALING through the evolutionarily conserved insulin pathway is critical for organismal homeostasis, controlling everything from growth regulation and development to metabolic homeostasis through glucose and lipid metabolism. In mammals, insulin synthesis and release takes place in pancreatic β-cells. Insulin production is regulated by many factors such as nutrient status and hormonal signals (Newsholme et al. 2010). After translation, insulin is packaged into dense-core vesicles (DCVs) and trafficked to the plasma membrane. Transport of insulin-containing DCVs is microtubule dependent, and the microtubule motor kinesin-1 is known to influence insulin granule transport (Meng et al. 1997; Tabei et al. 2013). DCV transport is additionally regulated by Rab27a. Through its effectors Slac2c, Noc2, Slp4, Exophilin8, and coronin3, Rab27a regulates movement of DCVs and their docking and fusion to the plasma membrane (Yi et al. 2002; Kasai et al. 2005; Kimura et al. 2008; Kimura and Niki 2011; Wang et al. 2013). DCV release is modulated largely via glucose stimulation and internalization, resulting in increased β-cell ATP levels. This induces the closure of ATP-dependent potassium channels and cell depolarization, triggering an influx of calcium ions through voltage-dependent calcium channels. Ca2+ promotes formation of the SNARE complex, allowing DCV fusion and insulin release (Kasai et al. 2010). Thus, proper packaging, trafficking, and exocytosis of insulin-containing DCVs is central to regulating insulin secretion. Defects in insulin production and trafficking arise early in the pathogenesis of diabetes. Many factors involved in DCV trafficking and the molecular details of DCV release remain elusive.
Research in animal models, in particular in Drosophila with its large genetic toolkit and fast generation time, can provide mechanistic insights into insulin-like peptide (ILP) production and DCV transport and release. Drosophila ILPs are homologous to human and mouse insulin/insulin-like growth factors (Brogiolo et al. 2001). Deletion of Ilps 1-5 results in smaller flies with lower metabolic activity (Zhang et al. 2009), while ubiquitous overexpression of Ilp2 is sufficient to promote growth (Ikeya et al. 2002). In flies, ILPs are produced and secreted mainly by insulin-producing cells (IPCs) in the brain to control growth and metabolism (Ikeya et al. 2002; Rulifson et al. 2002). ILP secretion is dependent on autonomous regulation and on inputs received from other cellular populations (Colombani et al. 2003; Geminard et al. 2009; Bai et al. 2012; Rajan and Perrimon 2012). ILPs are also produced by fat body cells during the pupal nonfeeding stages (Okamoto et al. 2009; Slaidina et al. 2009). Flies that lack IPCs have delayed development, reduced growth, and increased circulating sugar levels (Rulifson et al. 2002), suggesting that IPCs in flies play a role comparable to β-cells in mammals.
IPCs number only 14 of ∼100,000 neurons. They develop from a single pair of neuroblasts in the anterior neuroectoderm during late embryogenesis (Wang et al. 2007). During larval stages, IPCs secrete ILPs to promote growth and regulate sugar metabolism, while concurrently undergoing morphological development. Although the morphological development of IPCs during larval stages has not been well characterized, their neuronal processes extend through the brain to the aorta and the corpora cardiac compartment of the ring gland for ILP release (Rulifson et al. 2002). Adult IPCs are important for regulating starvation resistance, responding to oxidative and temperature stress, and adjusting carbohydrate and lipid metabolism (Nassel 2012).
The long neurites of larval and adult IPCs suggest that ILPs require extensive intracellular transport to reach secretion sites, the mechanism of which is largely unexplored. To identify additional cellular components that are important for insulin secretion in vivo, we sequenced messenger RNA (mRNA) purified from IPCs and identified IPC-enriched gene expression. To obtain a relatively pure population of fly IPCs for mRNA extraction, we used laser-capture microdissection (LCM). Compared with other cell- and tissue-specific RNA isolation techniques for Drosophila, including FACS (Tirouvanziam et al. 2004), magnetic bead-based cell purification (Iyer et al. 2009), and RNA-binding protein-based strategies (Miller et al. 2009), LCM has advantages for isolating specific cell types, especially for cells that are clustered, like IPCs. LCM has a reasonably high degree of spatial resolution and accuracy (Iyer and Cox 2010). We first characterized the temporal development of IPCs in detail and analyzed the transcriptome of early third instar IPCs. We identified 193 genes as enriched in IPCs, in comparison to randomly captured neurons, and found that many orthologous genes are active in mammalian pancreatic β-cells. In parallel, we tested 31 YFP-tagged dominant-negative (DN) Rab proteins (Zhang et al. 2007) for genes involved in ILP transport. Rab proteins, members of the family of Ras-like GTPases, control many cellular trafficking paths (Stenmark 2009). Our two approaches identified two genes essential in Drosophila for proper trafficking and secretion of ILP-containing DCVs. Here we identify Rab1 as necessary in IPCs to modulate ILP secretion. We discovered a novel function for Unc-104/Kif1a, a kinesin-3 microtubule motor previously known to transport synaptic vesicles (Okada et al. 1995; Pack-Chung et al. 2007; Barkus et al. 2008), in the transport of DCVs along the axons of IPCs.
Materials and Methods
Fly strains and genetics
Fly lines used are listed in Supporting Information, Table S6. The temperature shift for Gal80ts experiments was conducted in the following way: Embryos were collected on a molasses cap within a 4-hr period at 25° followed by 40-hr incubation at 18°. Thirty newly hatched first instar larvae were then transferred to each vial, kept at 18° for another 4 days, and shifted to 29° for 24 hr before dissection. Fly food (2×: 3.4% yeast, 8.3% cornmeal, 6% sucrose, 1% agar) was used to raise flies (Geminard et al. 2009).
Laser microdissection
The method was adapted from Spletter et al. (2007). Early third instar larval brains were dissected and quickly aligned and frozen with optimal cutting temperature compound (Tissue Tek). We sectioned from the posterior end of the mouth hook to the posterior end of the brain lobes, the position of which was marked by the anterior end of a pupa at one end of the block. A dehydration series (distilled H2O for 1 min, 50% ethanol for 30 sec, 70% ethanol for 30 sec, 95% ethanol for 30 sec, 100% ethanol for 30 sec, 100% ethanol for 2 min, xylene for 2 min, xylene for 5 min) was conducted before each section (10 μm thick) was examined under fluorescence with a 20× objective to identify sections that contain IPCs. IPCs were marked by expression of a genetically encoded GFP reporter (an Ilp2-Gal4 driving a UAS-mCD8-GFP reporter gene). In addition, UAS-nuclear red fluorescent protein (RFP) was included in the same fly line to independently label IPC cell bodies with RFP. This double labeling of IPCs, together with strong expression of mCD8-GFP (two copies) in these neurons, significantly sped up the process and reliability of IPC identification in cryosections. IPC-enriched samples were captured into PCR tube caps on a Leica LCM microscope (model ASLMD) using Laser Microdissection software version 4.4.
RNA isolation, amplification, and mRNA sequencing library construction
Total RNA of laser-captured samples was extracted using Arcturus PicoPure RNA Isolation Kit (Molecular Devices). mRNA was amplified in two rounds using the Arcturus RiboAmp HS PLUS Amplication Kit (Molecular Devices). Amplified mRNA from each sample was fragmented to 100–200 nt using 10× RNA fragmentation buffer (Ambion). Fragmented RNAs were ligated to the 3′ linker (“Linker-1,” IDT Inc.) and 5′ linker [5′-ACGCTCTTCCGATCTv-3′ (uppercase, DNA; v, barcodes with four RNA molecules: cugg, cguc, acuu, or cccu (IDT Inc.)]. Complementary DNA (cDNA) was amplified with 18 PCR cycles, using forward primer 5′-GAT ACG GCG ACC ACC GAG ATC TAC ACT CTT TCC CTA CAC GAC GCT CTT CCG ATC T-3′ and reverse primer 5′-CAA GCA GAA GAC GGC ATA CGA GCT CTT CCG ATC TAT TGA TGG TGC CTA CAG-3′ to produce sequencing libraries for the Illumina GA II sequencing system. The details of the RNA library construction protocol are available in File S5.
For whole brain (Elav-Gal4 > w1118), total RNA from third instar larval brains was extracted using Trizol (Invitrogen) following the manufacturer’s instructions. No mRNA amplification was used. mRNA sequencing libraries were constructed using a TruSeq RNA sample preparation kit (Illumina).
Mapping sequencing reads and mRNA profiling
Barcode splitting was performed on the FASTQ file. The first four bases of each read were compared to the barcodes, and up to one mismatch was allowed in the barcode bases. After barcode splitting and read segregation, each mRNA sample was aligned using tophat (version 2.0.0) (Trapnell et al. 2009), with default parameters against the reference Drosophila melanogaster genome and transcriptome [dm3/BDGP Release 5, from the University of California at Santa Cruz (UCSC) Genome Browser] (Adams et al. 2000; Fujita et al. 2011). Next, cuffdiff in the cufflinks package was used to determine differentially expressed genes and transcripts between the IPC replicates and the control replicates (Trapnell et al. 2010), where Reads Per Kilobase of exon model per Million mapped reads (RPKM) was calculated for each gene/transcript in each cell type and compared with each other.
Database for Annotation, Visualization and Integrated Discovery functional annotation analysis
Functional annotation of 193 IPC-enriched transcripts was carried out using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 (Huang da et al. 2009a,b). Entrez_IDs of the 193 genes were used as input. A total of 191 of 193 Entrez_IDs were annotated as known Drosophila genes by DAVID. Among the 191 known Drosophila genes, 115 (60.2%) genes have biological function annotations in the GOTERM_BP_FAT database. Annotation categories were searched and clustered using GOTERM_BP_FAT databases. Biological functional clusters were ranked based on an enrichment score, which was statistically measured by Fisher’s exact test in DAVID.
Searching mouse homologs of fly genes
Mouse homologs of IPC-enriched genes were found in the National Center for Biotechnology Information (NCBI) HomoloGene Database Release 66 (http://www.ncbi.nlm.nih.gov/homologene/). This method identified mouse orthologs of 104 IPC-enriched genes (193 in total). In addition, five homolog pairs (Ilp2-Ins1, Ilp3-Ins1, Ilp5-Ins2, ia2-IA2, foxo-Foxo1) were added based on literature information (Brogiolo et al. 2001) and a BLAT (UCSC genome browser) homologous sequence search.
Fly tissue fixation and immunofluorescence
Fly brains or fat bodies were dissected in ice-cold PBS containing 0.1% Triton X-100 (PBST), and tissues (brains or fat bodies) were fixed in 4% paraformaldehyde for 20 min, followed by extensive washes with PBST. Five-percent normal goat serum (NGS) (Sigma, diluted in PBST) was added to fixed samples for 1 hr at room temperature for blocking. Subsequently, after washing out the NGS, primary antibodies [rat anti-ILP2 (Pierre Leopold); chick anti-GFP (Aves Labs); rabbit anti-mCherry/mtdTomato/dsRed (Clontech); rabbit anti-β-galactosidase (MP Biomedicals)] were diluted in 5% NGS and incubated with the samples at 4° overnight. After extensive washes with PBST, secondary antibodies (anti-rat Alexa 488, anti-rat Alexa633, anti-chick Alexa 488, and anti-rabbit Alexa 647) (Invitrogen) were diluted in 5% NGS and incubated with samples at 4° overnight. After PBST washes, samples were mounted in SlowFade Gold antifade reagent (Invitrogen) for slide preparation.
Fly weighing and pupae volume measurement
Embryos were collected on a molasses cap within a 4-hr period followed by a 24-hr incubation at 25°. Thirty newly hatched first instar larvae were then transferred to each vial and stayed at 29° until eclosion. Adult males (1–2 days after eclosion) from each vial (typically in groups of 10–20) were weighed on an analytic balance accurate to ±0.01 mg (Mettler Toledo). In the cases where balancer exists for the UAS-RNA interference (RNAi) lines or few adults emerged from each vial, males from multiple vials were combined for weighing.
Pupae were photographed. Pupae length (L) and diameter (D) were measured using ImageJ. Drosophila pupae are assumed to take the shape of an ellipsoid, and their volume could be estimated based on this shape. Pupae volume (V) was calculated using the following formula: V = (4/3) × 3.14159 × (D/2)2 × (L/2).
Trehalose and glucose measurement
Trehalose assay was performed as previously described (Rulifson et al. 2002). In brief, hemolymph—circulatory fluid in arthropods—from five to eight larvae was collected by tearing the cuticles and allowing the hemolymph to bleed out and pool on a siliconized glass slide. Hemolymph samples (0.5 μl) as well as trehalose and glucose standards were diluted in 100 μl of Infinity Glucose Reagent (Sigma), and 40 μl of the diluted hemolymph was mixed with 80 μl of Infinity Glucose Reagent with porcine trehalase added at 3 μl/ml in 96-well plates. After a 16- to 18-hr incubation at 37°, plates were read on a fluorescence plate reader (Bio-Tek Instruments model #FL600). Excitation was applied at 360 nm while emission occurred at 460 nm. Standard curves were generated for determining the combined glucose and trehalose concentration in hemolymph samples.
Quantitative RT-PCR
For each genotype, 3 × 10 third instar larval brains were collected and RNA was purified using RNAsy kit (Qiagen) according to the manufacturer’s instructions. RNA concentration was measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific). cDNA was synthesized from 1 μg RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative PCR (qPCR) reactions for each biological sample were carried out in duplicate using Taqman assays (Applied Biosystems) for Ilp2 and Rpl32. mRNA levels were compared between conditions using the ΔΔCt method.
Confocal microscopy and image quantifications
Fly and pupae images were captured using a Leica MZ FLIII fluorescenct dissecting microscope and a Leica DFC 500 camera. Images were acquired using a TCS SP2 confocal microscope (Leica) with a ×63/numerical aperture 1.4 oil-immersion objective. To quantify ILP2 levels, confocal Z-series of the IPCs were obtained at 1 μm step size with identical laser power and scanning settings between control and experimental samples. Image stacks were then projected into a Z-stack (sum intensity), and the mean fluorescence intensities across IPC cell bodies or fat body nuclei were measured using Image J (National Institutes of Health). For each larva, multiple brain IPCs were quantified and averaged. These averaged values were then used for statistics to estimate the variance between animals under the same genetic background. Error bars represent the standard error of the mean from at least three independent experiments.
Statistics
For fly weighing, pupae volume, trehalose level, IPC cell number, ILP fluorescence intensity, and qPCR results, data were represented as mean ± SEM. Student’s t-tests (two tailed, equal variance) were performed for statistical significance.
Results
IPC morphological changes during development
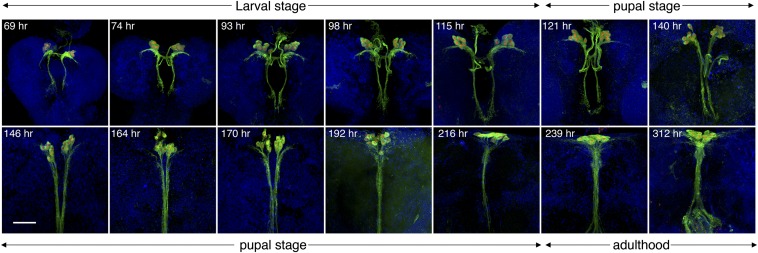
In preparation for laser dissection, we examined the developmental steps in the formation of IPCs to determine optimal conditions and timing for the dissection. Specification of fly brain IPCs during late embryogenesis has been characterized by several studies (Wang et al. 2007; Miguel-Aliaga et al. 2008; Hwang and Rulifson 2011), while post-specification developmental events such as neuronal morphogenesis of IPCs have been little studied. We followed the development of IPC morphology using Ilp2-Gal4-driven membrane (CD8) GFP (Figure 1 and File S1, File S2, File S3, and File S4). At early larval stages, IPCs exist as two symmetrical groups consisting of seven cells in each of the brain hemispheres. Their neuronal processes extend laterally and posteriorly within the brains, with some ending outside the brain, potentially on the aorta and the corpora cardiac compartment of the ring gland (Rulifson et al. 2002) (Figure 1 and File S2). At later larval stages, the cell bodies increase in size and their projections extend over greater distances. During pupal stages, the processes that had extended laterally from IPCs during larval stages are gradually dismantled. The processes that initially extended posteriorly from IPCs lengthen and eventually converge into one bundle (Figure 1 and File S3). At later pupal stages, the two IPC clusters converge to form one cell group near the midline. During adulthood new processes beneath IPCs are formed. These extend laterally with extensive arborizations. The posterior projection bundle becomes thickened, with extensive arborization at the terminals (Figure 1 and File S4). Larval IPCs are necessary for growth control and sugar homeostasis (Rulifson et al. 2002; Haselton and Fridell 2010). The adult IPCs, like larval IPCs, project to the corpora cardiac and to the aorta for ILP release (Rulifson et al. 2002; Kim and Rulifson 2004; Tatar 2004).
Figure 1.
IPC morphology changes during development. IPC neuronal structures were followed from early larval to adult stages. Developmental time points after embryo deposition are indicated. Green: Ilp2 > mCD8-GFP; red: Ilp2 > nuclear-RFP; blue: DAPI. up: anterior; down: posterior. Shown are maximum z-projections of confocal images taken at 1-μm step size. Flies were raised at 25° throughout development. Bar, 50 μm.
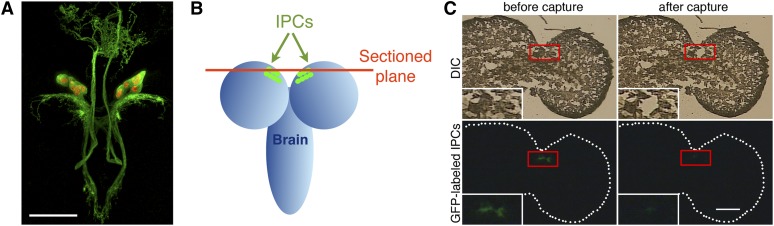
In the third instar, IPCs are clustered in two symmetrically organized groups of seven neurons (Figure 2A). The neurons’ structural characteristics and arrangement at this stage make them convenient for identification and laser capture. We chose early third instar larvae as the IPC source for two reasons: (1) the rapid growth during second and third instars is indicative of the need for IPC-secreted ILPs. (2) Neurite structures of IPCs do not change much during the third instar (Figure 1), making it more likely that active genes contribute to IPC-regulated ILP production rather than IPC neurite development.
Figure 2.
Capture of IPCs through laser microdissection. (A) Drosophila IPCs viewed by confocal microscopy. Green depicts IPC neuronal processes and cell bodies, and IPC cell nuclei are in red. (B) Schematic describing the cryosections that were chosen for IPC laser capture. (C) GFP-labeled IPCs before and after laser capture. Red boxes outline where IPCs are located and insets show higher magnifications of those regions. Dotted lines demarcate the boundary of the brain. Bars, 50 μm.
Amplifying sequences of mRNAs extracted from laser-captured larval IPCs
Frozen brain sections containing IPC cell bodies were identified using Ilp2-Gal4 > mCD8-GFP and Ilp2-Gal4 > nuclearRFP. During larval stages, the Ilp2-Gal4 driver is expressed at low levels in imaginal discs and at high levels in salivary glands and the 14 brain IPCs. In the brain, expression of the Ilp2-Gal4 drive is not detectable outside IPCs (Brogiolo et al. 2001; Rulifson et al. 2002). The successful capture of IPCs was demonstrated by the absence of GFP-labeled tissue from the residual sections (Figure 2, B and C). In total, 46 IPC cell bodies from 10 brains were captured and equally divided into two groups (IPC1 and IPC2). Two samples (control 1 and control 2) of ∼100 non-IPC, non-GFP cells each were collected from the same sections from which IPCs were captured. These adjacent regions of the brain are enriched in neurons that form the superior lateral, superior medial, and ventrolateral protocerebrum, dorsolateral neurons, mushroom body, optic tubercle, and lateral horn.
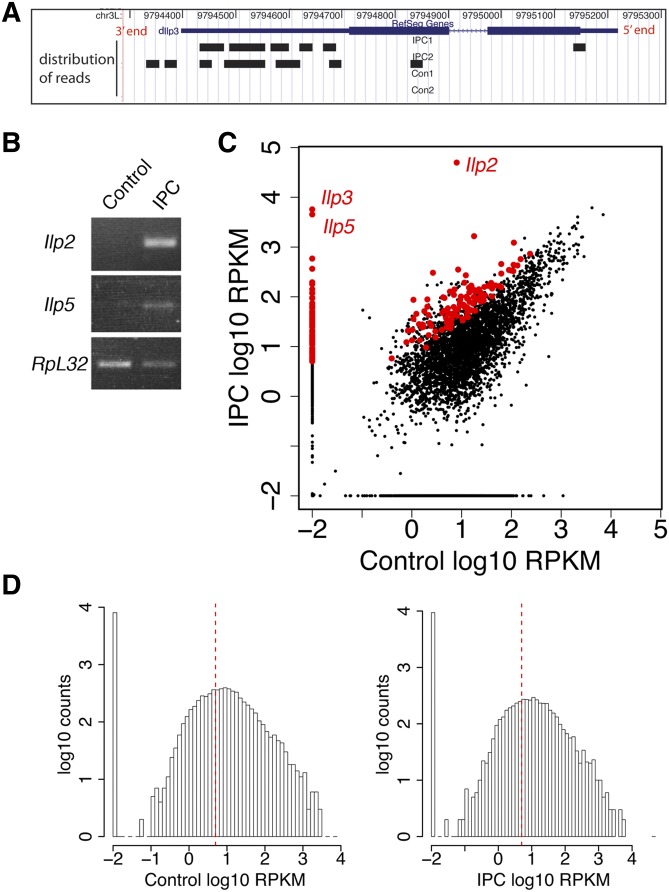
The small amount of RNA isolated from laser-captured cells necessitated amplification of the RNA sequences while trying to minimally skew relative mRNA abundances. We performed two rounds of RNA amplification from each of the four initial samples. In each round of RNA amplification, poly(A)RNA was reversed-transcribed to cDNA, which was then used as template for T7-based in vitro transcription to produce amplified RNA (Wang 2005). One limitation of this RNA amplification method is the lower 5′ representation of amplified RNA due to inefficient reverse transcription at each round of the amplification step (Baugh et al. 2001; Wang 2005) (Figure 3A). Two rounds of amplification may be insufficient for amplifying the low-copy-number mRNA species. Despite these potential problems, this amplification strategy produces a high correlation of gene expression profiles between unamplified RNA and RNA amplified from low-input RNA (500 pg of total RNA) (Van Gelder et al. 1990; Wang et al. 2000; Baugh et al. 2001; Lang et al. 2009). To evaluate the fidelity of the LCM and RNA amplification, we used RT-PCR to verify that the major larval Ilp mRNAs were enriched in amplified samples from captured IPCs, not in laser-dissected control samples (Figure 3B).
Figure 3.
mRNA sequencing of IPCs and control neurons. (A) A 3′ bias of mRNA sequencing reads. Distribution of mRNA-seq reads along Ilp3 mRNA as seen in the UCSC Genome Browser (Karolchik et al. 2003). RefSeq Gene track shows Ilp3 mRNA structure. Wide blue bar: exons; narrow blue bar: 5′ and 3′ untranslated region; blue line: intron. Distribution of mRNA reads from IPC1, IPC2, control 1, and control 2 samples is shown as black bars in individual tracks. (B) RT-PCR validation of laser-captured IPCs. Ilp2 and Ilp5 mRNA levels were assayed in amplified mRNAs from captured IPCs and control neural tissues. The housekeeping gene Ribosomal protein L32 (RpL32) was used as control. (C) Scatter plot of the mRNA-Seq expression data of IPCs and controls. Both the x- and the y-axis represent RPKM in log10 ratio. Red dots highlight IPC-enriched genes. A pseudo-count of 0.01 was added to RPKMs of all genes to avoid errors in log transformation of true. Thus, zero expression genes were represented as dots with expression of −2 in log10 on the plot. (D) Histograms of RPKM levels of genes expressed in controls and IPCs. The x-axis is log10 scale of gene expression measured in RPKM; the y-axis is gene counts in log10 scale. A pseudo-count of 0.01 was added to RPKMs of all genes to avoid errors in log transformation of true. The dashed red line represents RPKM = 5.
After aligning the sequencing reads to the reference D. melanogaster genome and transcriptome (dm3/BDGP Release 5 from UCSC genome browser, Table S1; Materials and Methods) (Adams et al. 2000; Fujita et al. 2011), we deduced the presence of transcripts representing 1851 genes that were common to both IPC samples and 2941 genes that were common to both control samples (RPKM ≥ 5.0 for moderate-to-high gene expression level) (Figure 3, C and D; Table S2). The number of expressed genes (total of 3373 from IPC and control samples) is 56% fewer than the number of genes detectably represented in third instar larval whole-brain mRNA-seq data (7704 genes, or 55% of the total fly genes). The lower transcriptome representation of our laser-dissected samples may be due to (1) the mRNAs from the 46 captured IPC cells and the ∼200 captured cells presumably from a subset of the genes that are expressed in the whole brain and (2) RNA degradation during LCM and/or insufficient mRNA amplification during IPC and control sample preparation.
Comparing IPCs to the control samples from adjacent regions, 1419 genes are expressed in both. A total of 432 genes are expressed only in IPC samples (IPC1 and IPC2), and 1522 genes are expressed only in control samples (control 1 and control 2). Among the 1419 shared genes, the majority showed little variance in gene expression level between IPCs and controls; most of them are clustered around the diagonal (Figure 3C; see also Table S2).
To select mRNAs that are more abundant in IPCs compared to control cells, we used the following criteria: (1) moderate-to-high expression level in IPCs (RPKM ≥5.0 in both IPC samples); (2) higher expression in IPCs than in controls for both replicates (both RPKMIPC1 and RPKMIPC2 must be greater than RPKMControl1 and RPKMControl2); (3) at least twofold enrichment of expression in IPCs compared to controls (RPKMIPC(average)/RPKMControl(average) ≥ 2.0); and (4) a statistically significant difference between expression in IPCs and expression in controls (P-value ≤ 0.15 by cufflinks/cuffdiff software).
In total, we detected 193 genes that were significantly enriched in IPCs compared to controls (Table S3). To identify the biological function groups represented by the IPC-enriched transcripts, we used DAVID, a gene functional classification tool (http://david.abcc.ncifcrf.gov). Genes were grouped into clusters based upon their gene ontology terms (GO terms) to identify genes sharing common biological functions (Table 1 and Table S4). Among the IPC-enriched transcripts analyzed by DAVID, the most enriched biological process cluster contains genes encoding the ILPs, G-protein-coupled receptors responding to hormone stimulation (Dh31-R, CCHa2-R, and mAChR), and other signaling transducers (Ggamma30A, Ras85D, Hrs, and drl). Genes in this group are essential for ILP production and could be required for IPCs to sense and process upstream neuronal/hormonal signals that act on IPCs. chico and foxo, both functioning in the ILP-receiving cells (Bohni et al. 1999; Puig et al. 2003), were enriched in IPCs, suggesting a possible autocrine role for ILPs to feed back to IPCs. The second most enriched biological process cluster contains genes encoding sugar metabolic enzymes: Pfk, Mdh2, Ald, Idh, Mdh1, CG9467, and CG8460. In mammalian β-cells, glucokinase and the glycolytic intermediates play important roles in glucose sensing (German 1993). Similarly, the enriched sugar metabolic enzymes in IPCs may participate in sugar sensing by IPCs.
Table 1. Top-ranked biological processes represented by IPC-enriched transcripts.
| FlyBase symbol | Gene name | |
|---|---|---|
| Enrichment score: 2.04 | ||
| Annotation cluster 1 | ||
| GO#0032868: response to insulin stimulus | Ilp2 | Insulin-like peptide 2 |
| GO#0032869: cellular response to insulin stimulus | Ilp3 | Insulin-like peptide 3 |
| GO#0043434: response to peptide hormone stimulus | Ilp5 | Insulin-like peptide 5 |
| GO#0008286: insulin-receptor-signaling pathway | chico | Insulin receptor substrate 1 |
| GO#0032870: cellular response to hormone stimulus | Ggamma30A | G-protein gamma 30A |
| GO#0007169: transmembrane receptor protein tyrosine-kinase-signaling pathway | SIFa | IFamide |
| Dh31 | Diuretic hormone 31 | |
| NPF | Neuropeptide F | |
| CCHa2-R | CCHamide-2 receptor | |
| Dh31-R | Diuretic hormone 31 receptor | |
| foxo | Forkhead box, subgroup O | |
| mAChR | Muscarinic acetylcholine receptor 60C | |
| Ms | Dromyosuppressin | |
| Hrs | Hepatocyte growth-factor-regulated tyrosine kinase substrate | |
| Mip | Myoinhibiting peptide precursor | |
| Ras85D | Ras oncogene at 85D | |
| drl | Derailed | |
| fog | Folded gastrulation | |
| Adar | Adenosine deaminase acting on RNA | |
| pix | Pixie | |
| Enrichment score: 1.67 | ||
| Annotation cluster 2 | ||
| GO#0006006: glucose metabolic process | Pfk | Phosphofructokinase |
| GO#0019318: hexose metabolic process | Mdh2 | Malate dehydrogenase 2 |
| GO#0005996: monosaccharide metabolic process | Ald | Aldolase |
| GO#0006096: glycolysis | Idh | Isocitrate dehydrogenase |
| GO#0006007: glucose catabolic process | Mdh1 | Malate dehydrogenase |
| GO#0019320: hexose catabolic process | CG9467 | Dmel_CG9467 |
| CG8460 | Dmel_CG8460 | |
| Vha26 | Vacuolar H[+]-ATPase 26kD E subunit | |
| Vha55 | Vacuolar H[+]-ATPase 55kD B subunit | |
| foxo | Forkhead box, subgroup O | |
| Enrichment score: 1.54 | ||
| Annotation cluster 3 | ||
| GO#0060538: skeletal muscle organ development | unc-104 | Kinesin-like protein unc-104 |
| GO#0048747: muscle fiber development | Frq1 | Frequenin 1 |
| GO#0007519: skeletal muscle tissue development | Gs2 | Glutamine synthetase 2 |
| GO#0014706: striated muscle tissue development | Vap-33-1 | Dmel_CG5014 |
| GO#0060537: muscle tissue development | drl | Derailed |
| GO#0050808: synapse organization | foxo | Forkhead box, subgroup O |
| Mef2 | Myocyte-enhancing factor 2 | |
Each annotation cluster represents GO terms with similar biological functions determined by DAVID. Biological functional clusters were ranked based on the enrichment score. The top six GO terms that have the lowest P-values (most enriched) within each cluster are shown. All genes involved in each cluster are shown. Full list of the GO terms of each cluster is in Table S5.
Unexpectedly, the third cluster of enriched biological function relates to “skeletal muscle organ development” (Table 1). Mef2 (Myocyte enhancer factor 2) is the only gene that is annotated as muscle-specific, while most other genes have annotated neuronal and muscular roles, among them neuromuscular junction development. Mef2 is a transcription factor that functions in both neuron and muscle development in Drosophila and is required to control circadian remodeling of clock neurons (Lilly et al. 1995; Sivachenko et al. 2013). The majority of the genes (unc-104, Frq1, Gs2, drl, Vap-33-1) in this cluster are involved in synapse assembly and/or synaptic transmission, but have a relatively lower enrichment score due to the presence of Mef2 in the list. IPCs are neurons, so we believed that “synapse organization” is a more proper representation of genes in cluster 3. IPCs presumably required such components for organizing IPC axonal terminals and for axonal transport needed for ILP release from these neurons.
Using HomoloGene from NCBI, we searched for mammalian homologs of fly IPC-enriched genes. Of the 193 IPC-enriched genes, 109 have clear mouse homologs (Table S5). Transcripts encoding Drosophila insulin-like peptides (Ilp2, -3, and -5) are the most enriched mRNAs in IPCs (>4000-fold compared with control neural tissue). This confirms that IPCs were included, and enriched, among the captured cells. mRNAs from amon and ia2, which encode Drosophila homologs of mammalian insulin-processing enzymes (Siekhaus and Fuller 1999; Rayburn et al. 2009) and insulin-secreting DCV components (Harashima et al. 2005; Kim et al. 2008), were also enriched (8- and 11-fold) in IPCs.
Screening for conserved genes that function in fly IPCs
Using available UAS-RNAi lines, 50 of the 193 IPC-enriched genes were tested for their influence on growth. Transgenes that encode RNAi were engineered to be active specifically in IPCs (Figure S1A). RNAi lines for individual genes from either the Vienna Drosophila RNAi Center (VDRC) or Transgenic RNAi Project (TRiP) collections were crossed with Ilp2-Gal4, and the adult progeny were examined for their weight (Dietzl et al. 2007; Ni et al. 2008) (Figure S1A). The Ilp2 promoter drives gene expression in IPCs starting during late embryogenesis and continuing into adulthood (Rulifson et al. 2002; Slaidina et al. 2009) (Figure 1). RNAi inhibition in IPCs of amon, foxo, Rab26, unc-104, hth, ald, Pkc98E, Vap-33-1, Vha26, and CG13506 had strong effects on adult fly size (>10% reduction) while inhibition of other genes had little or no effect (Figure S1A).
A recently published mRNA-seq database for genes expressed in adult mouse β-cells was used for cross-species comparison (Ku et al. 2012) (Table S5). In this study, the mouse homologs of amon, foxo, Pkc98E, Rab26, Vha26, unc-104, and Vap-33-1 had high expression levels in pancreatic β-cells compared with other cell types. Amon is the fly homolog of mammalian proprotein convertase subtilisin/kexin type 2 (PCSK2), a processing enzyme for prohormones and neuropeptide precursors. In β-cells, PCSK2 is involved in cleavage of proinsulin to insulin and C-peptide (Davidson et al. 1988). Foxo/Foxo1 has been implicated in β-cell proliferation in the mouse pancreas (Ai et al. 2010). In flies, Foxo functions with JNK to control Ilp transcription in response to oxidative stress (Hwangbo et al. 2004; Wang et al. 2005). PRKCE, the mouse homolog of fly Pkc98E, is associated with insulin granules for exocytosis upon inositol hexakisphosphate stimulation (Hoy et al. 2003; Mendez et al. 2003). Mouse Rab37, a homolog of fly Rab26, associates with insulin-secretory vesicles based on proteome analysis (Brunner et al. 2007), but its role in insulin secretion is unknown. Unc-104/Kif1a (a kinesin-3 microtubule motor), Vha26 (a proton transport ATPase), and Vap-33-1 (vesicle-associated membrane protein) have not been implicated in insulin secretion or β-cell proliferation. We chose Unc-104, a kinesin-3 microtubule motor, for further functional analysis, based upon the strong reduction in growth when it is inhibited in IPCs (Figure S1A).
Unc-104, a kinesin-3 family member, is required for neurite development and transporting ILP along the axons of IPCs
Depletion of unc-104 mRNA from IPCs during development dramatically reduced adult size (by 27% using a VDRC line and 12% using a TRiP line) (Figure S1A). Unc-104 was previously characterized as an anterograde motor that transports cargos, including neuropeptide-filled and synaptotagamin-bearing vesicles, in the fly nervous system (Pack-Chung et al. 2007; Barkus et al. 2008). Insulin is packed in DCVs before release (Dean 1973; Takahashi et al. 2004), so in IPCs the ILPs could be transported by Unc-104. To test this possibility, we first determined how ILPs are transported in normal IPCs.
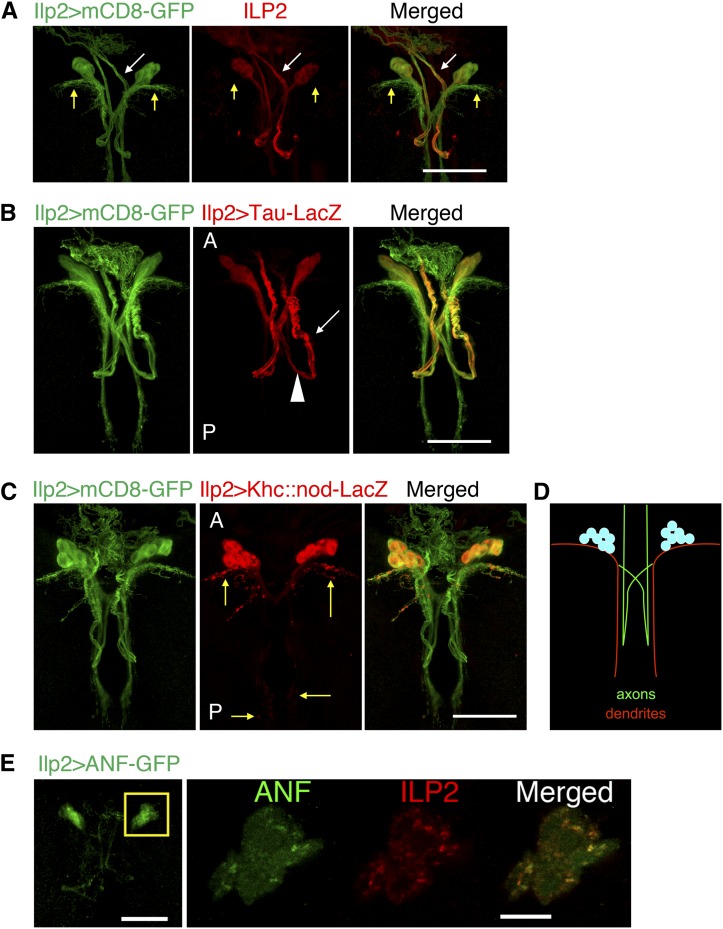
Immunofluorescence labeling of ILP2, one of the major ILPs, together with mCD8-labeled IPC neuronal processes showed that ILP2 is localized mainly in 14 cell bodies and some of their neurites (Figure 4A). To determine whether these ILP2-positive neurites have axon and/or dendrite features, we expressed axonal (Tau) and dendritic (Khc::nod) markers (Figure 4, B–D) (Rolls 2011). Tau-labeled IPC axonal bundles project contralaterally and posteriorly (Figure 4B, arrowhead), make a U-turn, and extend anteriorly (arrow) to terminals outside the brain. ILP2 resides mainly in these axonal projections (Figure 4A, white arrows; Figure 4B). The two main populations of Khc::nod-labeled dendritic arborizations extend laterally and posteriorly from the cell bodies (Figure 4C, yellow arrows). ILP2 was invisible, or at very low levels, in these neurite structures (Figure 4A, yellow arrows). The pattern of GFP-tagged DCV marker atrial natriuretic factor (ANF) in IPCs showed that DCV-carried neuropeptides are tightly associated with ILP2 granules in IPC cell bodies. This suggests that ILP2, like mammalian insulin, is packaged in DCVs for transport (Figure 4E) (Dean 1973; Rao et al. 2001; Takahashi et al. 2004). Since Ilp2 mRNA is made in the cell bodies (Brogiolo et al. 2001; Rulifson et al. 2002), ILP2 protein carried in DCVs is transported out of the cell bodies and along the axonal projections of IPCs.
Figure 4.
ILP2 is transported in DCVs along the axonal bundles in IPCs. (A) IPC neuronal structure from a third instar larval brain was labeled with Ilp2->mCD8-GFP. ILP2 localizes to some IPC neuronal projections (white arrow) but not to others (yellow arrows). (B–D) Localization of axonal (Tau) and dendritic (Khc::nod) markers in IPCs. In both figures, anterior (labeled with “A”) is toward the top and posterior (“P”) is toward the bottom. Arrowhead in B labels the portion of IPC axon bundles extending posteriorly from the cell bodies, and white arrow indicates, after the turn, IPC axons projecting anteriorly. Yellow arrows in C indicate the dendritic branches of IPCs. (D) Schematic of IPC polarity. (E) Localization of DCV in IPCs was examined by expressing exogenous ANF-GFP. Higher magnification of boxed region is shown for both ANF-GFP and ILP2 (single z optical section). Bars: 50 μm except in the zoomed-in image in E, where the bar represents 10 μm.
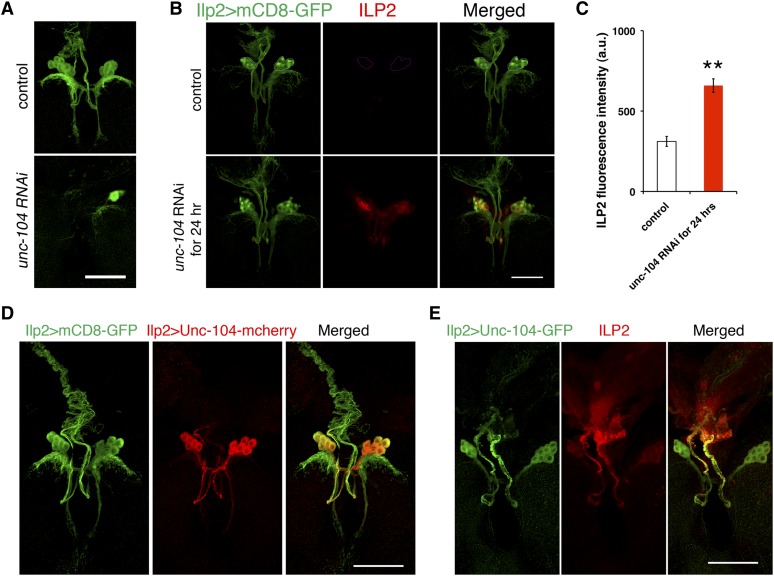
Depletion of Unc-104 from IPCs during early larval developmental stages reduced the number of IPCs, disrupted IPC morphology, and imposed ∼1 day of developmental delay in adult eclosion (Figure 5A), indicating that unc-104 is required during IPC development. Unc-104 is required for fly embryonic motor neuron synapse formation, larval synaptic terminal outgrowth, and dendrite morphogenesis of larval multidendritic neurons (Pack-Chung et al. 2007; Kern et al. 2013). In these past studies, a possible role of Unc-104 in insulin production would have been obscured by early developmental defects. To look specifically at Unc-104 function in ILP production, we employed tub-Gal80ts as a temporal Gal4 switch. Gal80ts inhibits Gal4 until increased temperature inactivates Gal80 and allows Gal4 to trigger gene transcription, in this case transcription of a gene encoding interfering unc-104 mRNA. We allowed Unc-104 to function through late larval development at 18°, by which time IPC neurite structure has fully developed (Figure 1), and then changed the temperature to 29° for 24 hr to induce production of unc-104 RNAi. This strategy successfully prevented occurrence of any visible defects in IPC development (Figure 5B). In control IPCs with functional Unc-104, low-level ILP2 was detected in the cell bodies. In contrast, 24-hr depletion of unc-104 mRNA during the second–third larval instar transition caused striking accumulation of ILP2 in IPC cell bodies, as well as enrichment of ILP2 in neurites extending from the IPC cell bodies (Figure 5B). Quantitation showed that reducing unc-104 function in IPCs caused a twofold increase in ILP2 in the cell bodies of IPCs (Figure 5C). qPCR analysis indicated no obvious increase in brain Ilp2 mRNA level after knockdown of unc-104 for 24 hr (Figure S2). Thus insulin secretion was likely inhibited after depletion of unc-104 mRNA.
Figure 5.
Unc-104 is localized to axons of IPCs and is required for IPC development and ILP2 secretion. (A) Control (Ilp2 > w1118, VDRC GD line control, #60000) and Ilp2 > unc-104 RNAi (VDRC line #23465) third instar brains were dissected. Ilp2 > mCD8-GFP (green) indicates the whole IPC neural structure. (B and C) ILP2 fluorescence intensities in the brain IPC cell bodies were compared between control (tubGal80ts, Ilp2 > w1118, VDRC GD line control, #60000, n = 9 brains) and unc-104 knockdown (n = 9 brains) in IPCs for 24 hr (tubGal80ts, Ilp2 > unc-104 RNAi). The images were taken with the same laser settings and manipulated in the same way. IPC cell bodies are outlined in the circle in control. (D and E) Localization of Unc-104 in IPCs was examined by expressing exogenous Unc-104-mCherry or Unc-104-GFP. Ilp2 > mCD8-GFP (green) indicates the whole IPC neural structure. Bars, 50 μm. Error bars: SEM, **P < 0.01.
Unc-104 is predominantly expressed in the nervous system in larval and adult flies (Chintapalli et al. 2007; Pack-Chung et al. 2007). To study the role of Unc-104 in IPCs, we examined the localization of Unc-104 protein in IPCs using tagged Unc-104 produced in IPCs (Ilp2 > Unc-104-mCherry, Figure 5D). Expression of this tagged Unc-104 in the nervous system is sufficient to rescue unc-104 mutant phenotypes (Pack-Chung et al. 2007; Kern et al. 2013). In IPCs, Unc-104-mCherry strongly colocalized with axons (Figure 5D). Unc-104-mCherry was also in IPC dendrites, but at a much lower level compared to axons. A similar pattern of Unc-104 localization was observed when GFP-tagged Unc-104 was expressed in IPCs (Figure 5E) (Barkus et al. 2008).
When unc-104 was depleted, ILP2 accumulated in IPC axons. The accumulation was limited to regions proximal to the cell bodies (Figure 5B, bottom). In view of the known role of Unc-104 in transporting DCVs in other neurons (Pack-Chung et al. 2007; Barkus et al. 2008), the localization of Unc-104 in IPCs and their axonal processes is consistent with a role for the motor protein in transporting ILP2 in DCVs along axons, especially in regions proximal to the cell bodies.
IPC-specific production of Rab DN proteins identifies Rab1 as a potent growth factor and hemolymph sugar modulator
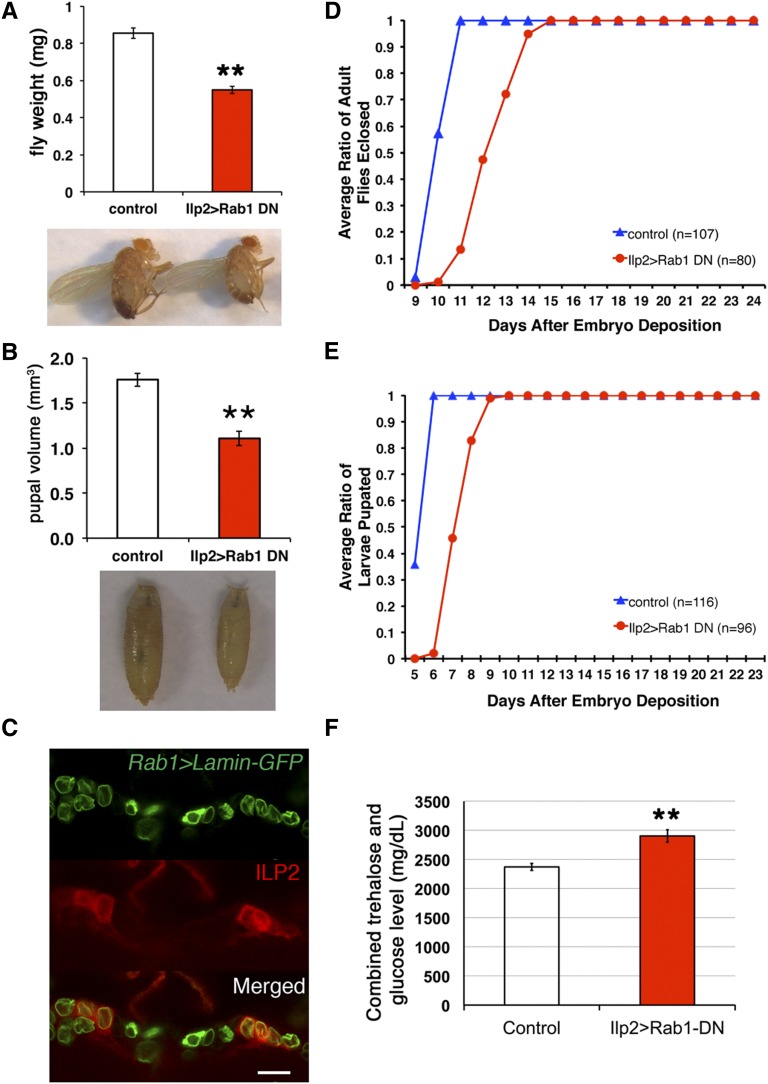
To identify additional proteins involved in fly ILP production, 29 dominant-negative fly Rab constructs (UAS-Rab DN) were screened by crossing at least one line for each Rab gene to the Ilp2-Gal4 driver (Rulifson et al. 2002; Zhang et al. 2007). Among 43 lines tested, only IPC-specific expression of Rab1-DN resulted in a dramatic (>40%) reduction in adult fly weight (Figure 6A and Figure S1B). The other Rab-DN lines, including Rab27-DN, had little effect on fly weights when expressed in IPCs (Figure S1B). Rab27 is the fly ortholog of mammalian Rab27a, which is involved in insulin granule exocytosis (Yi et al. 2002). In the larval brain lobes, Rab27 is expressed in the mushroom bodies and developing antennal lobes, but not in IPCs (Chan et al. 2011), which explains why expression of Rab27-DN in IPCs did not cause a growth phenotype. Expressing Rab26-DN in IPCs had a weaker effect on growth inhibition (7% reduction in size), compared with a 20% reduction in size achieved with Rab26 RNAi, suggesting that the DN construct is less effective than the RNAi construct (Figure S1). Rab1 is expressed ubiquitously in the brain including in all 14 IPCs (Figure 6C) (Chan et al. 2011). Producing Rab1-DN in IPCs dramatically reduced pupal size to only 61% of the controls, in keeping with the RNAi data (Figure 6B and Figure S3A).
Figure 6.
Rab1 is required in IPCs for normal animal growth and development. (A and B) Adult weights and pupal volume were compared between control flies (Ilp2-Gal4 > yw, n = 153 flies, n = 5 pupae) and flies expressing Rab1-DN in IPCs (Ilp2 > Rab1-DN, n = 138 flies, n = 6 pupae). (C) In a single confocal optical section, IPCs were labeled with anti-ILP2 antibody, and Rab1-expressed cells were labeled with Rab1 > Lamin-GFP. The other IPCs that were not displayed in this optical section also expressed Rab1. Bar, 20 μm. (D and E) Developmental time points of the onset of metamorphosis and adult eclosion were compared between control flies and flies expressing Rab1-DN in IPCs. (F) Combined trehalose and glucose level in the third instar larval hemolymph was compared between control (n = 12 pooled hemolymph samples) and Ilp2-Gal4 > Rab1-DN flies (n = 10 pooled hemolymph samples). Data were represented as mean ± SEM, **P < 0.01.
Reducing ILP production or secretion inhibits fly growth and developmental progression (Geminard et al. 2009; Zhang et al. 2009; Gronke et al. 2010). We tested whether inhibiting Rab1 in IPCs affects developmental timing. Flies producing Rab1-DN in IPCs eclosed and pupated, on average, ∼2 days later than control flies (Figure 6, D and E). One direct consequence of reduced ILP production or secretion is elevated levels of circulating sugars in hemolymph (Rulifson et al. 2002). With larvae producing Rab1-DN in IPCs, hemolymph levels of trehalose and glucose were elevated compared to control larvae (Figure 6F). The average combined trehalose and glucose levels for control larvae and Ilp2 > Rab1-DN larvae were 2372 and 2904 mg/dl, respectively. The values of carbohydrate concentration for the Rab1-DN larvae resembled the levels of IPC-ablated larvae (Rulifson et al. 2002). This elevated sugar level, together with developmental delay and growth inhibition caused by Rab1-DN produced in IPCs, suggests that Rab1 promotes ILP production by IPCs.
Rab1 is required for IPC dendrite morphogenesis and ILP transport out of the IPC cell bodies
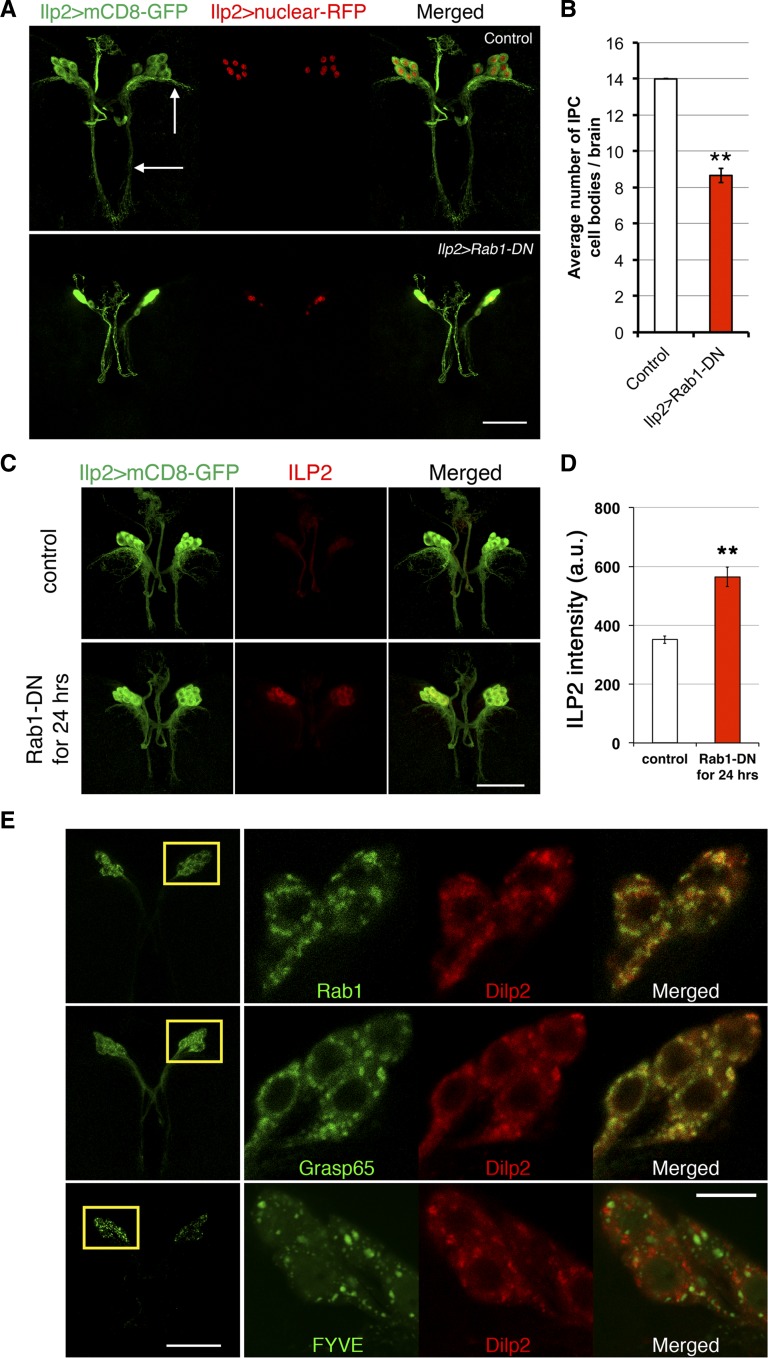
To explore the mechanism by which Rab1-DN inhibits ILP production, we first looked for any IPC developmental defects caused by constitutive inhibition of Rab1 in IPCs. IPC morphology and cell number were examined using mCD8-GFP and a RFP-conjugated nuclear marker, combined with Rab1-DN produced in the IPCs. Under these conditions, there were fewer IPCs in these larvae (Figure 7, A and B). The average IPC count was reduced from 14 ± 0.0 in wild-type larvae to 8.7 ± 0.4 in Ilp2 > Rab1-DN larvae. IPC neuronal morphology was dramatically disrupted. In Ilp2 > Rab1-DN larvae, dendritic arborizations were missing (Figure 7A, white arrows), while the axonal bundles were mostly intact. To specifically examine whether Rab1 controls ILP production, we employed the Gal80ts stretegy described earlier to inhibit Rab1 after IPC neurite structures had fully developed. As with unc-104 inhibition, late inhibition of Rab1 for 24 hr with the tub-Gal80ts system avoided IPC developmental defects but caused a 1.6-fold increase in ILP2 level in IPC cell bodies (Figure 7, C and D). The level of ILP2 was reduced in the axonal projections that connect the IPC cell bodies, suggesting that ILP2 was trapped in cell bodies rather than transported along axons. qPCR assays indicated no obvious changes in brain Ilp2 mRNA level after expression of Rab1-DN for 24 hr (Figure S2). As an alternative approach to inhibit Rab1 function, we expressed Rab1 RNAi using the Ilp2-Gal4 driver. At 29°, the RNAi caused strong accumulation of ILP2 in IPC cell bodies but left IPC morphology intact (Figure S3, B–D). Brains expressing Rab1 RNAi had the normal 14 IPCs, yet the RNAi reduced fly weight by 20%. The RNAi effect was milder than the effect of Rab1-DN (Figure S3A and Figure 6A). Rab1 RNAi in IPCs caused only a slight developmental delay (about one-half day delayed for pupation and adult eclosion) and allowed axons and dendrites of IPCs to be formed and maintained properly. There was some loss of left–right symmetry (Figure S3B), perhaps as a consequence of perturbing global growth. Despite the mild effects on development and IPC morphology, Rab1 RNAi caused a doubling of ILP2 accumulation in IPC cell bodies (Figure S3, C and D). Both types of depletion of Rab1 function suggest a role for Rab1 in controlling intracellular trafficking of ILP2.
Figure 7.
Rab1 colocalizes with ILP2 granules and is required for IPC development and ILP2 transport. (A) IPC neurite structure and nuclei were labeled with Ilp2 > mCD8-GFP and Ilp2 > nls-RFP in third instar larval brains. (Top) The white arrows indicate the dendrites of control IPCs (Ilp2-Gal4 > yw), which were missing in Ilp2-Gal4 > Rab1-DN brains. (B) Quantification of IPC cell numbers (based on Ilp2 > nls-RFP-labeled nuclei) indicated a reduction (five to six) in Ilp2-Gal4 > Rab1-DN brains (n = 9 brains; for controls, n = 10). (C and D) ILP2 fluorescence intensities in the brain IPC cell bodies were compared between control (tubGal80ts, Ilp2 > yw, n = 10 brains) and Rab1 inhibition (n = 14 brains) in IPCs for 24 hr (tubGal80ts, Ilp2 > Rab1-DN). The images were taken with the same laser settings and manipulated in the same way. (E) Labeling of Rab1 (Ilp2 > Rab1-YFP), a Golgi marker (Ilp2 > Grasp65-GFP), or an endosome marker (Ilp2 > FYVE-GFP) in combination with ILP2 staining. The left column (bottom magnification) shows the localization of GFP/YFP-tagged proteins as a projection of z-stacks. Rab1- and FYVE-labeled endosomes were localized almost exclusively in the cell bodies, while Grasp65-labeled cis-Golgi distributed in both the IPC cell bodies and the axons. Boxed regions are shown in higher magnification in the right columns. Rab1-YFP, Grasp-GFP, FYVE-GFP, and ILP2 all had punctate localization patterns in IPCs. For examination of colocalization between vesicles/granules, single z optical sections were shown. Bars, 50 μm except in the zoomed-in images in E, where the bar represents 10 μm. Error bars: SEM, **P < 0.01.
When Rab1 is inhibited in IPCs, ILP secretion is inhibited during transit from cell bodies to the axonal tracts. To investigate whether Rab1 directly influences ILP transport in IPCs, ILP2 was labeled with antibodies in wild-type IPCs expressing Rab1-YFP. Rab1-YFP was localized within IPC cell bodies with very low fluorescence in IPC axons or dendrites (Figure 7E). Rab1-YFP and ILP2 immunofluorescent labeling displayed punctate patterns in IPC cell bodies. Strikingly, the majority of ILP2 punctae overlapped with Rab1 punctae, suggesting that Rab1-containing vesicles directly transport ILP granules along the route of ILP secretion.
Rab1 controls ER-to-Golgi transport (Stenmark 2009), so we investigated whether ILP2 granules reside in the Golgi. Producing the GFP-labeled Golgi marker Grasp65 in IPCs revealed punctate Golgi patterns that colocalized with ILP2 granules (Figure 7E). As a negative control, the endosome marker FYVE-GFP was produced in IPCs. The FYVE-labeled endosomes were in a punctate pattern, like Grasp65-GFP and Rab1-YFP, but had few overlaps with ILP2 granules. These data, together with the elevated ILP levels in IPCs after Rab1 inhibition, suggested that ILP is delivered by Rab1 to the Golgi in IPC cell bodies. The failed delivery of ILPs outside the cell bodies, due to Rab1 inhibition, results in failed secretion.
Discussion
Drosophila brain IPCs regulate development, growth, metabolism, stress resistance, life span, feeding, locomotor activity, olfactory sensitivity, sleep, and ethanol sensitivity in response to internal physiological and external nutritional signals (Nassel 2012). Until now, only a handful of genes were known to function in IPCs. By combining LCM, RNA-seq, two UAS-RNAi libraries, and our UAS-Rab-DN collection, we systematically screened for genes that are important for IPC function. Among genes that rendered IPC-specific phenotypes when inhibited in IPCs, we focused on unc-104 and Rab1 and investigated their roles in ILP secretion and IPC development.
Conservation between mammalian pancreatic β-cells and fly IPCs
Among the neurons, glial cells, intestinal muscle cells, adipocytes, and salivary gland cells that can produce ILPs in flies (Brogiolo et al. 2001; Ikeya et al. 2002; Miguel-Aliaga et al. 2008; Okamoto et al. 2009; Chell and Brand 2010; O’Brien et al. 2011; Sousa-Nunes et al. 2011), brain IPCs are the best-characterized. Several lines of evidence indicate that Drosophila brain IPCs and mammalian pancreatic β-cells share functional and physiological similarities: (1) Genetic ablation of fly IPCs or deletion of fly ILPs results in metabolic phenotypes similar to mammals with β-cell/insulin deficiency (Brogiolo et al. 2001; Ikeya et al. 2002; Rulifson et al. 2002; Zhang et al. 2009; Gronke et al. 2010). (2) Fly ILPs and mammalian insulin carry out their functions by activating a conserved signaling pathway in target tissues (Baker and Thummel 2007). (3) In Drosophila, brain IPCs and adipokinetic hormone-producing cells are physically connected through neuronal projections and in this sense at least are functionally analogous to pancreatic β- and α-cells (Kim and Rulifson 2004). (4) β-Cells and fly IPCs respond to Leptin/Leptin-like cytokine secreted from adipose tissue to regulate ILP release (Kieffer et al. 1997; Rajan and Perrimon 2012).
Phylogenetic analyses of insulin-producing cells suggest that mammalian β-cells and fly IPCs have common evolutionary origins or have different origins but have undergone convergent evolution (Arntfield and Van Der Kooy 2011). Mammalian β-cells arise from the endoderm (Jensen 2004), and fly IPCs are neurons arising from the ectoderm (Wang et al. 2007). Fly IPCs have long axons requiring extensive transport of vesicles, while β-cells have no axons. Yet phylogenic analyses of insulin-expressing cells indicate that neurons evolved to secrete insulin before there were β-cells, creating a puzzle about the evolutionary origin of β-cells (Arntfield and Van Der Kooy 2011). In jellyfish, insulin is produced only by neurons (Davidson et al. 1971). In Caenorhabditis elegans and in Drosophila, insulin is produced by neurons and by endoderm-derived cells (Brogiolo et al. 2001; Rulifson et al. 2002; Li et al. 2003). The insulin-producing cells in a worm’s nervous system and intestine respond to food intake to control development, life span, and stress resistance (Li et al. 2003; Iser et al. 2007).
Pancreatic β-cells have some similarities to neurons. From the perspective of physiology, (1) β cells and neurons pack their signaling peptides into secretory granules and release them using action potentials (Rorsman and Renstrom 2003). (2) Hypothalamic neurons can sense blood glucose levels (Lam et al. 2005), as β-cells do. From the perspective of gene expression, (1) β-cells express certain “neural-specific” genes, which encode sodium channels, neurofilaments, neurotransmitters (such as GABA), and their receptors (Escurat et al. 1991; Philipson et al. 1993; Glassmeier et al. 1998; Adeghate and Ponery 2002; Xu et al. 2006). (2) Neurons and β-cells do not express the gene encoding the neuron-restrictive silencing factor/repressor silencing transcription factor, a negative regulator of neuron fate that is made only in non-neuronal cells (Atouf et al. 1997). For all these reasons, β-cells and insulin-expressing neurons may share properties and proteins useful for controlling insulin production and secretion.
By analyzing the IPC transcriptome using LCM and mRNA sequencing, we found a group of IPC-enriched mRNAs that are orthologs of mammalian genes active during insulin production and secretion. Larval-stage-specific Ilps (Ilp2, Ilp3, and Ilp5) were found, as expected, and we detected enrichment of amon, ia2, and Pkc98E mRNAs, which encode Drosophila orthologs of key mammalian insulin-processing enzymes, DCV components, and signal transducers that control insulin secretion (Settle et al. 1995; Siekhaus and Fuller 1999; Hoy et al. 2003; Mendez et al. 2003; Dong et al. 2006; Rayburn et al. 2009). Many other IPC-enriched genes have mouse orthologs that are preferentially transcribed in pancreatic β-cells (Ku et al. 2012). This provided good evidence that our captured samples were highly enriched in IPCs and strengthened the evidence for conservation between fly IPCs and mammalian β-cells. Functional tests indicated that at least 20% (10 of 50) of the IPC-enriched genes that have mammalian orthologs are required in IPCs for proper Drosophila body size to be attained. RNAi with expression of any of these genes in IPCs strongly (>10%) reduced growth. Therefore, the combination of LCM and RNA-seq is a powerful and sensitive method for characterizing the transcriptome of a specific cell type, such as IPCs, that is present in limited cell numbers in the brain.
In addition to ILPs, four neuropeptide genes were enriched in our IPC samples: Myoinhibiting peptide precursor (Mip), Dromyosuppressin (Dms), neuropeptide F (npf), and IFamide (IFa). These may be contaminants from adjacent cells (Park et al. 2008). Since our starting materials were only 23 captured cells for each IPC sample, if one of the neuropeptide mRNAs is at a higher level (e.g., >100-fold) compared to average brain cells, it may have been purified along with IPC mRNAs. Since we used third instar larval IPCs for transcriptome analysis, mRNAs for G-protein-coupled receptors, membrane channels, and transporter proteins that are abundant in IPCs earlier in development may not be present among our enriched mRNAs. Larvae undergo developmental transitions in the third instar stage that require ecdysone and involve metabolic changes. By capturing gene expression during that time we sampled a larger range of cell properties and conditions than doing the same analysis of adult IPCs.
IPC neuronal polarity and neurite development
Our studies and prior work suggest that ILP2 is synthesized in IPC cell bodies and transported out of the cell bodies mainly along Tau-labeled axonal projections to IPC axonal terminals, where ILP2 is released (Brogiolo et al. 2001; Ikeya et al. 2002; Rulifson et al. 2002; De Velasco et al. 2007; Geminard et al. 2009). ILP2 is also released within the brain (Bader et al. 2013). By following IPCs during larval development, we observed that IPC axons and dendrites extend over greater distances until the early third instar larval stage. The neurite extension of IPCs is required to compensate for the size increase of brain lobes, so that connections between IPCs and other brain regions such as subesophageal ganglion are maintained (Rulifson et al. 2002; De Velasco et al. 2007). IPC dendrite extension and more extensive dendritic arborization might also be required to form new neuronal connections with other neurons, thus adjusting to new developmental and physiological needs.
Unc-104 in ILP transport in IPCs
Two regulators of ILP production, Unc-104 and Rab1, emerged as the strongest regulators from our screens. Strict regulation of ILP secretion is required to adjust downstream insulin signaling to food availability and metabolic status. Precise control is achieved through regulatory steps in IPCs: sensing of neuronal or hormonal signals, control of ILP secretion machinery by the signals, directed transport of ILPs to the IPC axonal terminal, and final release of ILP out of IPCs. Based upon our experiments, both Unc-104 and Rab1 are involved in directed transport of ILPs.
Unc-104 in worms and flies, and its mammalian homolog Kif1a, are anterograde motor proteins that transport DCVs along axons (Okada et al. 1995; Zahn et al. 2004; Pack-Chung et al. 2007; Barkus et al. 2008; Lo et al. 2011). DCVs exist in different cell types. In neurons, DCVs are responsible for transporting, processing, and secreting neuropeptide cargos. In pancreatic β-cells, insulin is packed into DCVs. The movement and secretion of insulin-containing DCVs to β-cell surfaces is a Ca2+-dependent process that requires kinesin heavy chain movement along microtubules (Meng et al. 1997; Donelan et al. 2002; Cui et al. 2011). It is currently unknown whether other kinesin family proteins contribute to insulin-containing DCV movement and secretion. Our study shows that a kinesin 3 family protein, Unc-104, transports insulin granules along the axons of IPCs. ILP2 colocalizes with DCVs in IPC cell bodies. We observed Unc-104 and ILP2 distributed along IPC axons. Reducing unc-104 function using two different small interfering RNAs (siRNAs) caused accumulation of ILP2 in cell bodies and in their proximal axonal projections. Consistent with a role for fly Unc-104 in transporting insulin-like peptides, C. elegans Unc-104 transports fluorescently tagged insulin-like protein 22 and IA-2 in motor neurons (Goodwin et al. 2012). Mammalian Kif1a’s role in insulin transport and secretion has not been reported. Kif1a mutant mice die soon after they are born due to severe motor and sensory defects (Yonekawa et al. 1998). They exhibit defects in the localization of synaptic vesicle precursors. Whether newborn Kif1a mutant mice have diabetic symptoms has not been reported.
Rab1 in ILP transport in IPCs
The small GTPase Rab1 regulates membrane trafficking within early Golgi compartments and in the ER–Golgi transition. In Drosophila, Rab1 was first described as a contributor in the maintenance of photoreceptor cell structure by mediating vesicle transport between the rough ER and Golgi body (Satoh et al. 1997). Our study shows that Rab1 is expressed ubiqutously in the fly brain, including IPCs. The specific effect with only Rab1-DN but not other Rab-DNs when expressed in IPCs shows that Rab1-DN interferes specifically with endogenous Rab1 function. It also rules out an alternative interpretation that Rab1-DN titrates some endogenous protein such as a GEF that works on multiple Rabs, one of which is the real regulator, in addition to Rab1. Inhibiting Rab1 function through dominant-negative or siRNA constructs caused accumulation of ILP2 in cell bodies and substantially reduced ILP2 in IPC axons. YFP-tagged Rab1 is localized predominantly in the cell bodies and exhibits a tight association with ILP2 granules cytologically. A recent study of the silkworm Bombyx mori showed that Rab1 is restricted to a small number of neurons in the brain, where it colocalizes with Bombyxin, an insulin family peptide, in the pars intercerebralis area (Uno et al. 2013). Proteomic analysis showed that Rab1 is enriched in immunopurified β-cell insulin granules (Hickey et al. 2009). Rab1 may be conserved as a critical molecule needed for insulin production.
Unc-104 and Rab1 in IPC development
In addition to their roles in ILP production, Unc-104 and Rab1 are required for IPC development. Constitutive depletion of unc-104 mRNA from IPCs using Ilp2-Gal4 resulted in severe disruption of IPC axons and dendrites and dramatically reduced IPC cell numbers as detected by Ilp2 > mCD8-GFP. Since IPCs are post-mitotic neurosecretory cells and Ilp2 mRNA is produced only after IPC differentiation (Wang et al. 2007; Hwang and Rulifson 2011), Ilp2-Gal4-driven unc-104 RNAi does not interfere with the initial formation and specification of IPCs during late embryogenesis. Therefore, the observed reduction in IPC cell numbers at late larval stages comes from cell death during larval development. This phenotype is consistent with the observation that Kif1a mutant mice, and cultures of Kif1a mutant neurons, exhibit marked neuronal degeneration and death, which could be caused by insufficient neural stimulation due to disrupted neural connections (Yonekawa et al. 1998). Given the roles that Unc-104 plays in fly motor neuron and multidendritic neuron development (Pack-Chung et al. 2007; Kern et al. 2013), the axonal and dendritic morphology disruption seen in IPCs could be an initial neurite outgrowth defect, a later maintenance defect, or a combination of both.
We have found that constitutive inhibition of Rab1 in IPCs, using a dominant-negative protein, resulted in disruption of IPC dendrites to a lesser extent than unc-104 RNAi. Rab1 functions in ER-to-Golgi transport, so this preferential disruption of IPC dendrites over IPC axons could be explained if IPC dendrites, like those of fly “da” neurons and rodent hippocampal neurons, are more sensitive to the reduction of ER-to-Golgi transport than IPC axons (Ye et al. 2007). Rab1-DN induced cell death during IPC development, although less severely than unc104 RNAi. The milder phenotypes with respect to neuron viability and neurite morphology in IPCs producing Rab1-DN compared to IPCs expressing unc-104 RNAi are consistent; a milder defect in neurite morphology may underlie a milder defect in neuron viability. Alternatively, Rab1-DN might cause accumulation of cytotoxic components such as misfolded α-synucleins in IPCs, which could underlie the loss of IPCs (Cooper et al. 2006).
Proteins like Rab1 and Unc-104 have multiple functions in a variety of cell types. Using cell-type-specific interference, we have explored their roles in production and transport of insulin-like peptides and determined the cellular and whole-organism phenotypes associated with their damaged functions. These proteins join an important list of critical factors needed for the controlled production and release of insulin-family proteins.
Supplementary Material
Acknowledgments
We thank the Transgenic RNAi Project at Harvard Medical School (National Institutes of Health/National Institute of General Medical Sciences R01-GM084947), the Vienna Drosophila Research Center, and the Bloomington Stock Center for providing transgenic RNAi and other fly stocks used in this study. We appreciate the generous help from Pierre Leopold, Eric Rulifson, Ping Shen, Tom Schwarz, Bill Saxton, Robin Hiesinger, and Liqun Luo, who provided flies and antibodies; Liqun Luo for supporting M.L.S. and Ziming Weng, Phil Lacrout, and Arend Sidow in flow-cell preparation, sequencing, and data processing; Sam Gu for help and suggestions on preparing mRNA-seq libraries for sequencing; Kaye Suyama, Weiwei Chen, and Dan Gui for helping with fly stock maintenance; and Virginia Walbot for sharing with us the cryostat and LCM in her lab. We also thank Liqun Luo, Tom Hartl, Ljiljana Milenkovic, and Alex Brown for critical comments on the manuscript. Research in the Kim lab was supported by the Howard Hughes Medical Institute (HHMI). J.C. was supported by Stanford University’s Bio-X Interdisciplinary Initiatives Seed Grant. J.N. is a postdoctoral fellow, and S.K.K. and M.P.S. are Investigators of the HHMI.
Footnotes
Communicating editor: N. Perrimon
Literature Cited
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., et al. , 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Adeghate E., Ponery A. S., 2002. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34: 1–6. [DOI] [PubMed] [Google Scholar]
- Ai J., Duan J., Lv X., Chen M., Yang Q., et al. , 2010. Overexpression of FoxO1 causes proliferation of cultured pancreatic beta cells exposed to low nutrition. Biochemistry 49: 218–225. [DOI] [PubMed] [Google Scholar]
- Arntfield M. E., van der Kooy D., 2011. β-Cell evolution: how the pancreas borrowed from the brain: the shared toolbox of genes expressed by neural and pancreatic endocrine cells may reflect their evolutionary relationship. Bioessays 33: 582–587. [DOI] [PubMed] [Google Scholar]
- Atouf F., Czernichow P., Scharfmann R., 1997. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J. Biol. Chem. 272: 1929–1934. [DOI] [PubMed] [Google Scholar]
- Bader R., Sarraf-Zadeh L., Peters M., Moderau N., Stocker H., et al. , 2013. The IGFBP7 homolog Imp-L2 promotes insulin signaling in distinct neurons of the Drosophila brain. J. Cell Sci. 126: 2571–2576. [DOI] [PubMed] [Google Scholar]
- Bai H., Kang P., Tatar M., 2012. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 11: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. D., Thummel C. S., 2007. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 6: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus R. V., Klyachko O., Horiuchi D., Dickson B. J., Saxton W. M., 2008. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol. Biol. Cell 19: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh L. R., Hill A. A., Brown E. L., Hunter C. P., 2001. Quantitative analysis of mRNA amplification by in vitro transcription. Nucleic Acids Res. 29: E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni R., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., et al. , 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell 97: 865–875. [DOI] [PubMed] [Google Scholar]
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., et al. , 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11: 213–221. [DOI] [PubMed] [Google Scholar]
- Brunner Y., Coute Y., Iezzi M., Foti M., Fukuda M., et al. , 2007. Proteomics analysis of insulin secretory granules. Mol. Cell. Proteomics 6: 1007–1017. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Scoggin S., Wang D., Cherry S., Dembo T., et al. , 2011. Systematic discovery of Rab GTPases with synaptic functions in Drosophila. Curr. Biol. 21: 1704–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell J. M., Brand A. H., 2010. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 143: 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Colombani J., Raisin S., Pantalacci S., Radimerski T., Montagne J., et al. , 2003. A nutrient sensor mechanism controls Drosophila growth. Cell 114: 739–749. [DOI] [PubMed] [Google Scholar]
- Cooper A. A., Gitler A. D., Cashikar A., Haynes C. M., Hill K. J., et al. , 2006. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Wang Z., Cheng Q., Lin R., Zhang X. M., et al. , 2011. Targeted inactivation of kinesin-1 in pancreatic beta-cells in vivo leads to insulin secretory deficiency. Diabetes 60: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W., Rhodes C. J., Hutton J. C., 1988. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature 333: 93–96. [DOI] [PubMed] [Google Scholar]
- Davidson J. K., Falkmer S., Mehrotra B. K., Wilson S., 1971. Insulin assays and light microscopical studies of digestive organs in protostomian and deuterostomian species and in coelenterates. Gen. Comp. Endocrinol. 17: 388–401. [DOI] [PubMed] [Google Scholar]
- Dean P. M., 1973. Ultrastructural morphometry of the pancreatic β-cell. Diabetologia 9: 115–119. [DOI] [PubMed] [Google Scholar]
- de Velasco B., Erclik T., Shy D., Sclafani J., Lipshitz H., et al. , 2007. Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev. Biol. 302: 309–323. [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- Donelan M. J., Morfini G., Julyan R., Sommers S., Hays L., et al. , 2002. Ca2+-dependent dephosphorylation of kinesin heavy chain on beta-granules in pancreatic beta-cells. Implications for regulated beta-granule transport and insulin exocytosis. J. Biol. Chem. 277: 24232–24242. [DOI] [PubMed] [Google Scholar]
- Dong H., Kumar M., Zhang Y., Gyulkhandanyan A., Xiang Y. Y., et al. , 2006. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia 49: 697–705. [DOI] [PubMed] [Google Scholar]
- Escurat M., Djabali K., Huc C., Landon F., Becourt C., et al. , 1991. Origin of the beta cells of the islets of Langerhans is further questioned by the expression of neuronal intermediate filament proteins, peripherin and NF-L, in the rat insulinoma RIN5F cell line. Dev. Neurosci. 13: 424–432. [DOI] [PubMed] [Google Scholar]
- Fujita P. A., Rhead B., Zweig A. S., Hinrichs A. S., Karolchik D., et al. , 2011. The UCSC Genome Browser database: update 2011. Nucleic Acids Res. 39: D876–D882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geminard C., Rulifson E. J., Leopold P., 2009. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 10: 199–207. [DOI] [PubMed] [Google Scholar]
- German M. S., 1993. Glucose sensing in pancreatic islet beta cells: the key role of glucokinase and the glycolytic intermediates. Proc. Natl. Acad. Sci. USA 90: 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassmeier G., Hopfner M., Riecken E. O., Mann B., Buhr H., et al. , 1998. Inhibition of L-type calcium channels by somatostatins in human neuroendocrine tumor cells of the gut. Ann. N. Y. Acad. Sci. 859: 208–209. [DOI] [PubMed] [Google Scholar]
- Goodwin P. R., Sasaki J. M., Juo P., 2012. Cyclin-dependent kinase 5 regulates the polarized trafficking of neuropeptide-containing dense-core vesicles in Caenorhabditis elegans motor neurons. J. Neurosci. 32: 8158–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke S., Clarke D. F., Broughton S., Andrews T. D., Partridge L., 2010. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6: e1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S., Clark A., Christie M. R., Notkins A. L., 2005. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc. Natl. Acad. Sci. USA 102: 8704–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton A. T., Fridell Y. W., 2010. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Albany, NY) 2: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey A. J., Bradley J. W., Skea G. L., Middleditch M. J., Buchanan C. M., et al. , 2009. Proteins associated with immunopurified granules from a model pancreatic islet beta-cell system: proteomic snapshot of an endocrine secretory granule. J. Proteome Res. 8: 178–186. [DOI] [PubMed] [Google Scholar]
- Hoy M., Berggren P. O., Gromada J., 2003. Involvement of protein kinase C-epsilon in inositol hexakisphosphate-induced exocytosis in mouse pancreatic beta-cells. J. Biol. Chem. 278: 35168–35171. [DOI] [PubMed] [Google Scholar]
- Huang da, W., B. T. Sherman, and R. A. Lempicki, 2009a Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da, W., B. T. Sherman, and R. A. Lempicki, 2009b Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4: 44–57. [DOI] [PubMed] [Google Scholar]
- Hwang H. J., Rulifson E., 2011. Serial specification of diverse neuroblast identities from a neurogenic placode by Notch and Egfr signaling. Development 138: 2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo D. S., Gershman B., Tu M. P., Palmer M., Tatar M., 2004. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429: 562–566. [DOI] [PubMed] [Google Scholar]
- Ikeya T., Galic M., Belawat P., Nairz K., Hafen E., 2002. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 12: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Iser W. B., Gami M. S., Wolkow C. A., 2007. Insulin signaling in Caenorhabditis elegans regulates both endocrine-like and cell-autonomous outputs. Dev. Biol. 303: 434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer E. P., Cox D. N., 2010. Laser capture microdissection of Drosophila peripheral neurons. J. Vis. Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, E. P., S. C. Iyer, M. J. Sulkowski, and D. N. Cox, 2009 Isolation and purification of Drosophila peripheral neurons by magnetic bead sorting. J. Vis. Exp., No. 34: pii: 1599. [DOI] [PMC free article] [PubMed]
- Jensen J., 2004. Gene regulatory factors in pancreatic development. Dev. Dyn. 229: 176–200. [DOI] [PubMed] [Google Scholar]
- Karolchik D., Baertsch R., Diekhans M., Furey T. S., Hinrichs A., et al. , 2003. The UCSC Genome Browser Database. Nucleic Acids Res. 31: 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Hatakeyama H., Ohno M., Takahashi N., 2010. Exocytosis in islet beta-cells. Adv. Exp. Med. Biol. 654: 305–338. [DOI] [PubMed] [Google Scholar]
- Kasai K., Ohara-Imaizumi M., Takahashi N., Mizutani S., Zhao S., et al. , 2005. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J. Clin. Invest. 115: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J. V., Zhang Y. V., Kramer S., Brenman J. E., Rasse T. M., 2013. The kinesin-3, unc-104 regulates dendrite morphogenesis and synaptic development in Drosophila. Genetics 195: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer T. J., Heller R. S., Leech C. A., Holz G. G., Habener J. F., 1997. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes 46: 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Bang H., Ko S., Jung I., Hong H., et al. , 2008. Drosophila ia2 modulates secretion of insulin-like peptide. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151: 180–184. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Rulifson E. J., 2004. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431: 316–320. [DOI] [PubMed] [Google Scholar]
- Kimura T., Niki I., 2011. Rab27a in pancreatic beta-cells, a busy protein in membrane trafficking. Prog. Biophys. Mol. Biol. 107: 219–223. [DOI] [PubMed] [Google Scholar]
- Kimura T., Kaneko Y., Yamada S., Ishihara H., Senda T., et al. , 2008. The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J. Cell Sci. 121: 3092–3098. [DOI] [PubMed] [Google Scholar]
- Ku G. M., Kim H., Vaughn I. W., Hangauer M. J., Oh C. M., et al. , 2012. Research resource: RNA-Seq reveals unique features of the pancreatic β-cell transcriptome. Mol. Endocrinol. 26: 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T. K., Gutierrez-Juarez R., Pocai A., Rossetti L., 2005. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 309: 943–947. [DOI] [PubMed] [Google Scholar]
- Lang J. E., Magbanua M. J., Scott J. H., Makrigiorgos G. M., Wang G., et al. , 2009. A comparison of RNA amplification techniques at sub-nanogram input concentration. BMC Genomics 10: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Kennedy S. G., Ruvkun G., 2003. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 17: 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly B., Zhao B., Ranganayakulu G., Paterson B. M., Schulz R. A., et al. , 1995. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science 267: 688–693. [DOI] [PubMed] [Google Scholar]
- Lo K. Y., Kuzmin A., Unger S. M., Petersen J. D., Silverman M. A., 2011. KIF1A is the primary anterograde motor protein required for the axonal transport of dense-core vesicles in cultured hippocampal neurons. Neurosci. Lett. 491: 168–173. [DOI] [PubMed] [Google Scholar]
- Mendez C. F., Leibiger I. B., Leibiger B., Hoy M., Gromada J., et al. , 2003. Rapid association of protein kinase C-epsilon with insulin granules is essential for insulin exocytosis. J. Biol. Chem. 278: 44753–44757. [DOI] [PubMed] [Google Scholar]
- Meng Y. X., Wilson G. W., Avery M. C., Varden C. H., Balczon R., 1997. Suppression of the expression of a pancreatic beta-cell form of the kinesin heavy chain by antisense oligonucleotides inhibits insulin secretion from primary cultures of mouse beta-cells. Endocrinology 138: 1979–1987. [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I., Thor S., Gould A. P., 2008. Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biol. 6: e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R., Robinson K. J., Cleary M. D., Doe C. Q., 2009. TU-tagging: cell type-specific RNA isolation from intact complex tissues. Nat. Methods 6: 439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel D. R., 2012. Insulin-producing cells and their regulation in physiology and behavior of Drosophila. Can. J. Zool. 90: 476–488. [Google Scholar]
- Newsholme P., Gaudel C., McClenaghan N. H., 2010. Nutrient regulation of insulin secretion and beta-cell functional integrity. Adv. Exp. Med. Biol. 654: 91–114. [DOI] [PubMed] [Google Scholar]
- Ni J. Q., Markstein M., Binari R., Pfeiffer B., Liu L. P., et al. , 2008. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5: 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien L. E., Soliman S. S., Li X., Bilder D., 2011. Altered modes of stem cell division drive adaptive intestinal growth. Cell 147: 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Yamazaki H., Sekine-Aizawa Y., Hirokawa N., 1995. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81: 769–780. [DOI] [PubMed] [Google Scholar]
- Okamoto N., Yamanaka N., Yagi Y., Nishida Y., Kataoka H., et al. , 2009. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev. Cell 17: 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack-Chung E., Kurshan P. T., Dickman D. K., Schwarz T. L., 2007. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat. Neurosci. 10: 980–989. [DOI] [PubMed] [Google Scholar]
- Park D., Veenstra J. A., Park J. H., Taghert P. H., 2008. Mapping peptidergic cells in Drosophila: where DIMM fits in. PLoS ONE 3: e1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L. H., Kusnetsov A., Larson T., Zeng Y., Westermark G., 1993. Human, rodent, and canine pancreatic beta-cells express a sodium channel alpha 1-subunit related to a fetal brain isoform. Diabetes 42: 1372–1377. [DOI] [PubMed] [Google Scholar]
- Puig O., Marr M. T., Ruhf M. L., Tjian R., 2003. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 17: 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A., Perrimon N., 2012. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151: 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Lang C., Levitan E. S., Deitcher D. L., 2001. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 49: 159–172. [DOI] [PubMed] [Google Scholar]
- Rayburn L. Y., Rhea J., Jocoy S. R., Bender M., 2009. The proprotein convertase amontillado (amon) is required during Drosophila pupal development. Dev. Biol. 333: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls M. M., 2011. Neuronal polarity in Drosophila: sorting out axons and dendrites. Dev. Neurobiol. 71: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Renstrom E., 2003. Insulin granule dynamics in pancreatic beta cells. Diabetologia 46: 1029–1045. [DOI] [PubMed] [Google Scholar]
- Rulifson E. J., Kim S. K., Nusse R., 2002. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296: 1118–1120. [DOI] [PubMed] [Google Scholar]
- Satoh A., Tokunaga F., Kawamura S., Ozaki K., 1997. In situ inhibition of vesicle transport and protein processing in the dominant negative Rab1 mutant of Drosophila. J. Cell Sci. 110(Pt 23): 2943–2953. [DOI] [PubMed] [Google Scholar]
- Settle S. H., Jr, Green M. M., Burtis K. C., 1995. The silver gene of Drosophila melanogaster encodes multiple carboxypeptidases similar to mammalian prohormone-processing enzymes. Proc. Natl. Acad. Sci. USA 92: 9470–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekhaus D. E., Fuller R. S., 1999. A role for amontillado, the Drosophila homolog of the neuropeptide precursor processing protease PC2, in triggering hatching behavior. J. Neurosci. 19: 6942–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivachenko A., Li Y., Abruzzi K. C., Rosbash M., 2013. The transcription factor Mef2 links the Drosophila core clock to Fas2, neuronal morphology, and circadian behavior. Neuron 79: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M., Delanoue R., Gronke S., Partridge L., Leopold P., 2009. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev. Cell 17: 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Nunes R., Yee L. L., Gould A. P., 2011. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471: 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spletter M. L., Liu J., Su H., Giniger E., Komiyama T., et al. , 2007. Lola regulates Drosophila olfactory projection neuron identity and targeting specificity. Neural Dev. 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., 2009. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10: 513–525. [DOI] [PubMed] [Google Scholar]
- Tabei S. M., Burov S., Kim H. Y., Kuznetsov A., Huynh T., et al. , 2013. Intracellular transport of insulin granules is a subordinated random walk. Proc. Natl. Acad. Sci. USA 110: 4911–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Hatakeyama H., Okado H., Miwa A., Kishimoto T., et al. , 2004. Sequential exocytosis of insulin granules is associated with redistribution of SNAP25. J. Cell Biol. 165: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M., 2004. The neuroendocrine regulation of Drosophila aging. Exp. Gerontol. 39: 1745–1750. [DOI] [PubMed] [Google Scholar]
- Tirouvanziam R., Davidson C. J., Lipsick J. S., Herzenberg L. A., 2004. Fluorescence-activated cell sorting (FACS) of Drosophila hemocytes reveals important functional similarities to mammalian leukocytes. Proc. Natl. Acad. Sci. USA 101: 2912–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., et al. , 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno T., Sakamoto K., Isoyama Y., Hiragaki S., Uno Y., et al. , 2013. Relationship between the expression of Rab family GTPases and neuropeptide hormones in the brain of Bombyx mori. Histochem. Cell Biol. 139: 299–308. [DOI] [PubMed] [Google Scholar]
- Van Gelder R. N., von Zastrow M. E., Yool A., Dement W. C., Barchas J. D., et al. , 1990. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc. Natl. Acad. Sci. USA 87: 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., 2005. RNA amplification for successful gene profiling analysis. J. Transl. Med. 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Miller L. D., Ohnmacht G. A., Liu E. T., Marincola F. M., 2000. High-fidelity mRNA amplification for gene profiling. Nat. Biotechnol. 18: 457–459. [DOI] [PubMed] [Google Scholar]
- Wang H., Ishizaki R., Xu J., Kasai K., Kobayashi E., et al. , 2013. The Rab27a effector exophilin7 promotes fusion of secretory granules that have not been docked to the plasma membrane. Mol. Biol. Cell 24: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. C., Bohmann D., Jasper H., 2005. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121: 115–125. [DOI] [PubMed] [Google Scholar]
- Wang S., Tulina N., Carlin D. L., Rulifson E. J., 2007. The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proc. Natl. Acad. Sci. USA 104: 19873–19878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E., Kumar M., Zhang Y., Ju W., Obata T., et al. , 2006. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 3: 47–58. [DOI] [PubMed] [Google Scholar]
- Ye B., Zhang Y., Song W., Younger S. H., Jan L. Y., et al. , 2007. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130: 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z., Yokota H., Torii S., Aoki T., Hosaka M., et al. , 2002. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol. Cell. Biol. 22: 1858–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa Y., Harada A., Okada Y., Funakoshi T., Kanai Y., et al. , 1998. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell Biol. 141: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn T. R., Angleson J. K., MacMorris M. A., Domke E., Hutton J. F., et al. , 2004. Dense core vesicle dynamics in Caenorhabditis elegans neurons and the role of kinesin UNC-104. Traffic 5: 544–559. [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu J., Li C. R., Momen B., Kohanski R. A., et al. , 2009. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 106: 19617–19622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Schulze K. L., Hiesinger P. R., Suyama K., Wang S., et al. , 2007. Thirty-one flavors of Drosophila rab proteins. Genetics 176: 1307–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.