Abstract
Evolutionary forces shape patterns of genetic diversity within populations and contribute to phenotypic variation. In particular, recurrent positive selection has attracted significant interest in both theoretical and empirical studies. However, most existing theoretical models of recurrent positive selection cannot easily incorporate realistic confounding effects such as interference between selected sites, arbitrary selection schemes, and complicated demographic processes. It is possible to quantify the effects of arbitrarily complex evolutionary models by performing forward population genetic simulations, but forward simulations can be computationally prohibitive for large population sizes (>105). A common approach for overcoming these computational limitations is rescaling of the most computationally expensive parameters, especially population size. Here, we show that ad hoc approaches to parameter rescaling under the recurrent hitchhiking model do not always provide sufficiently accurate dynamics, potentially skewing patterns of diversity in simulated DNA sequences. We derive an extension of the recurrent hitchhiking model that is appropriate for strong selection in small population sizes and use it to develop a method for parameter rescaling that provides the best possible computational performance for a given error tolerance. We perform a detailed theoretical analysis of the robustness of rescaling across the parameter space. Finally, we apply our rescaling algorithms to parameters that were previously inferred for Drosophila and discuss practical considerations such as interference between selected sites.
Keywords: forward simulations, recurrent selection, rescaling
A central goal of population genetics is to determine the strength and rate of natural selection in populations. Natural selection affects patterns of genetic diversity within populations and is likely to influence phenotypes of biological and medical interest (Bustamante et al. 2005; Torgerson et al. 2009; Maher et al. 2012; Arbiza et al. 2013). There exists a large body of literature focused on mathematical models of selection in populations and inferring the action of selection on DNA sequences under these models (recent reviews include Pool et al. 2010; Crisci et al. 2012; Cutter and Payseur 2013). One such model is known as recurrent hitchhiking, in which patterns of diversity at a selectively neutral locus are altered due to repeated positive selection at linked loci.
Recurrent hitchhiking has been theoretically explored (Smith and Haigh 1974; Ota and Kimura 1975; Kaplan et al. 1989; Stephan et al. 2006; Coop and Ralph 2012) and applied to DNA sequences of various organisms (Bachtrog 2008; Jensen et al. 2008; Ingvarsson 2010; Singh et al. 2013). The classic work of Stephan et al. (1992) modeled the dynamics of the neutral locus in a single sweep with diffusion-based differential equations, which they solved approximately. Wiehe and Stephan (1993) later showed that their solution for single sweeps could be applied to a recurrent sweep model, where the expected reduction in neutral diversity is well approximated by , where α = 2Ns, N is the population size, s is the selection coefficient, r is the recombination rate, λ is the rate of positively selected substitutions, and I is a constant that approximates the value of an integral. However, little work has been done to explore recurrent sweeps with forward simulations (but see Kim and Stephan 2003 and Chevin et al. 2008, where interfering substitutions were studied with forward simulations, and the discussion herein).
It is crucial to understand the dynamics of recurrent sweeps (and other population genetic models) when realistic perturbations to the model are introduced, which is often difficult in a coalescent framework. In contrast, with forward simulations it is straightforward to introduce arbitrarily complex models, including demographic processes, interference between selected sites, simultaneous negative and positive selection, and variable strength of selection or recombination rate across a chromosome. Furthermore, forward simulations can be performed exactly under a given model, and hence they can be used as a direct test of theoretical predictions. Simulations can be used in conjunction with inference methods such as approximate Bayesian computation to estimate parameters when the likelihood function of the data under the model is unknown (Beaumont et al. 2002).
In population genetics, forward methods have often been overlooked in favor of reverse time coalescent simulators due to computational efficiency (Hernandez 2008) (for an overview of coalescent and forward simulation techniques, see Kim and Wiehe 2009). Although coalescent simulations are generally more computationally efficient, in most applications they require some a priori knowledge of allele trajectories. Recent improvements in computer memory and processor speeds have made forward simulations more tractable. However, simulations of recurrent hitchhiking in some parameter regimes of interest (e.g., N > 105) are still computationally prohibitive, so it is frequently necessary to rescale model parameters (e.g., N and chromosome length, L) (Kim and Wiehe 2009). Currently, the literature provides some guidelines for performing parameter scaling in forward simulations (Hoggart et al. 2007), but it is not clear that these methods will be generally applicable to all models or hold in all parameter regimes.
In this investigation, we examine recurrent sweeps through forward simulation and theory. We provide a detailed, practical discussion of simulations of recurrent sweeps in a forward context, focusing on scaling laws of relevant parameters such as N, λ, r, α, and L. We evaluate a “naive” parameter rescaling algorithm and show that this technique can bias patterns of variation in the simulations because it is not conservative with respect to the underlying genealogical process, particularly in the large α, small N regime. We quantify the effect of large values of the selection coefficient s on recurrent hitchhiking through theory. Finally, we leverage these principles to make gains in computational efficiency with a simple algorithm that provides the best possible performance for a prespecified error threshold and apply the method to simulations of parameters previously inferred in Drosophila.
Model
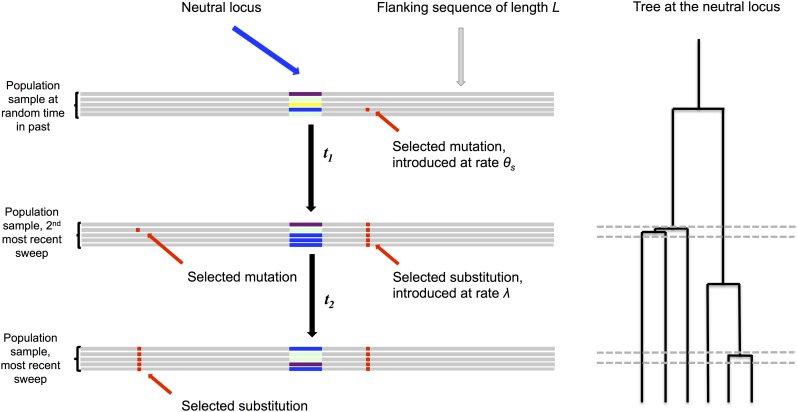
Here, we describe the recurrent hitchhiking model (shown schematically in Figure 1), upon which we build the results and simulations in this article. Key parameters of the model are discussed below and summarized in Table 1.
Figure 1.
A pictorial representation of the recurrent hitchhiking model. Diverse neutral haplotypes are indicated with various colors at the neutral locus. When a selected site is introduced and eventually goes to fixation, it drags linked neutral variation to higher frequency. Selected mutations can occur at any distance from the neutral locus within the flanking sequence. Viewed from the perspective of the genealogy, sweep events generate an excess of recent common ancestry at a linked neutral site, reducing the overall height of the coalescent tree relative to neutrality. Selected sites that are more closely linked to the neutral site have a stronger impact on the overall height of the tree because they induce more common ancestry on a short timescale. The overall impact of linked selection at a neutral locus is a function of the strength of selection, the rate of recombination, and the rate at which selected sites reach fixation. The neutral site is assumed to be nonrecombining, but this assumption can be relaxed in simulations.
Table 1. Parameter definitions.
| N | Population size |
| τf | Time of fixation of selected allele |
| t | Time in generations |
| x(t) | Frequency of the selected allele |
| h(t) | Relative heterozygosity at the neutral locus among selected chromosomes |
| ql | Minor allele frequency at site l |
| l0 | Length of neutral region |
| L | Flanking sequence length |
| π | Nucleotide diversity, |
| π0 | Nucleotide diversity under neutrality |
| πN | Nucleotide diversity in a population of size N |
| p(t) | Probability of common ancestry at the neutral locus |
| n | Number of sampled sequences |
| μ | Mutation rate per generation per chromosome per base pair |
| θ = 4Nμ | Population-scaled mutation rate |
| s | Selection coefficient |
| α = 2Ns | Population-scaled selection strength |
| r | Recombination rate per generation per chromosome per base pair |
| ρ = 4Nr | Population-scaled recombination rate |
| rf | Recombination fraction (probability of productive recombination, per generation per chromosome) |
| λ | Rate of positively selected substitutions per site per generation |
| kh | Rate of common ancestry induced by sweep events [see (4)] |
| Rc | Total rate of common ancestry induced by sweep and coalescent events |
| Iα,s | The integral [see (4)] |
| The integral [see (14)] | |
| I = 0.075 | A constant approximating Iα,s |
A neutral locus is flanked on both sides by sequences experiencing repeated positively selected substitutions at rate λ per generation per site. λ is assumed to be small enough that multiple positively selected mutations do not simultaneously sweep in the population and hence there is no interference (although interference between selected sites is not prohibited in the simulations performed herein). Population size is fixed at N. In forward simulations, there is no distinction between effective and census population size, so N = Ne. Recombination occurs at rate rf (recombination fraction) per generation per chromosome. Note that the recombination fraction is the probability that the number of recombination events between two loci is an odd number and cannot exceed 0.5. Each positively selected site has a selection coefficient . Heterozygous individuals have fitness 1 + s, while individuals homozygous for the selected allele have fitness 1 + 2s. The neutral locus itself is assumed to be nonrecombining. Any of the above constraints and model assumptions can be relaxed in forward simulations.
Consider the coalescent history at the neutral locus of two sequences sampled immediately after a selective sweep. If there is no recombination between the neutral and selected loci during the sweep, then the two sequences must share a common ancestor at some point during the sweep. If selection is sufficiently strong, then the time to fixation of selected alleles is effectively instantaneous relative to the neutral fixation process (Kaplan et al. 1989), and thus the expected heterozygosity at the neutral site at the completion of the sweep is nearly 0 because very few mutations are introduced during the sweep.
Recombination significantly complicates this model. Immediately after a sweep, the reduction in heterozygosity at the neutral locus is a function of the recombination distance between the selected substitution and the neutral locus and the strength of selection. Stephan, Wiehe, and Lenz calculated the reduction in heterozygosity at the neutral locus with a diffusion-based, differential equation framework (Stephan et al. 1992). They showed that the expected reduction in heterozygosity at the neutral locus among chromosomes carrying the selected allele, relative to the baseline heterozygosity, h(t), can be modeled with a simple differential equation, which they solved approximately.
Kaplan et al. (1989) showed that h(t) is closely related to the probability that two sequences sampled at the end of the sweep share a common ancestor at the neutral site during the sweep, p(t):
| (1) |
This allows the results of Stephan et al. (1992) to be interpreted in terms of the coalescent process at the neutral locus. Note that when t = τf (the end of the sweep), p(τf) represents the probability of common ancestry at the neutral locus for a pair of sequences at some point during the sweep because all chromosomes carry the selected allele at the end of a sweep. Throughout the article, we subscript variables of interest with τf to denote their values at the time of fixation and emphasize their dependence on the recombination fraction rf [e.g., ]. Rewriting results from Stephan et al. (1992) with (1), we obtain
| (2) |
where x(t) is the frequency of the selected allele at time t during the sweep. Equation 2 is equivalent to equation 5 of Barton (1998) when the selected allele is at low frequency.
Equation 2 can be interpreted in terms of the recombination process between the neutral and selected loci. In particular, there are two mechanisms that can change the proportion of selected sequences that share common ancestry at the neutral locus. The first term on the right-hand side (RHS) of (2) represents that chance of common ancestry in the previous generation among selected sequences that have already recombined off of the original background. The chance that any two such sequences share a common ancestor in the previous generation is . The second term represents the chance that a recombination event occurs between a selected chromosome and some nonselected chromosome, thereby reducing p(t). The first term is important only when the frequency of the selected site is low, because it is inversely proportional to the number of selected chromosomes, whereas the second term contributes nonnegligibly to the dynamics at all allele frequencies of the selected locus.
Consider the coalescent history at the neutral locus of two lineages sampled at the current time (not necessarily immediately after a sweep event). In each preceding generation, there is some chance that they share a common ancestor at the neutral locus due to normal coalescent events and some chance that they share common ancestry because of a sweep event. Since sweeps occur nearly instantaneously relative to the timescale of coalescence under neutrality, we can approximate the chance of common ancestry as two competing processes. Neutral events occur at rate and compete with sweep events, which happen at rate in a window of size drf, assuming that sweeps occur homogeneously across the chromosome and rf ≈ rL. Note that r and λ appear in a quotient in this rate, which implies that compensatory increases in the substitution and recombination rates cancel out. The factor of 2 represents the flanking sequence on either side of the neutral locus.
Following the results of Stephan et al. (1992), an approximate solution to (2) is
| (3) |
where Γ is the incomplete gamma function. Note that (3) is a function of rf, α, and s, but we denote only the dependence on rf since α and s are assumed to be fixed for the analysis herein.
Following Stephan et al. (1992), we denote the rate at which lineages merge due to sweep events as kh,
| (4) |
where is taken as the value of rf that corresponds to the end of the flanking sequence. If the flanking chromosome being modeled exceeds bp, previous work suggests that can be taken to be any value sufficiently far away from the neutral locus such that the value of kh is as close as desired to its asymptotic limit (Jensen et al. 2008). The factor of 2N is introduced to rescale in coalescent units, such that neutral coalescent events happen at rate 1.
The expectation of π in recurrent hitchhiking
In the recurrent hitchhiking (RHH) model, it is of great interest to describe the reduction in diversity as a function of the basic parameters of the model (α, r, λ, etc.). To make this dependence clearer, we perform two changes of variables in (4). First, we note that (4) was derived by Stephan et al. (1992) under the assumption that is small, such that the recombination fraction is given by rf ≈ rL. Here we are frequently concerned with values of rf that approach its maximum value of 0.5, which invalidates this approximation. We therefore rewrite (4) as a function of L, substituting for the quantity rf (Haldane 1919). We then substitute the quantity for L. Rewriting and kh as functions of u, we have
| (5) |
and
| (6) |
It is useful to examine the properties of (5) and (6) as a function of s. When s is small, (5) can be rewritten as
| (7) |
which removes the dependence on s and is identical to the quantity inside the integral on the RHS of equation 4 of Wiehe and Stephan (1993). Thus, the integral on the RHS of (6) is a function of the parameter α only when s is small. This is not necessarily the case as s becomes large, but we also note that (3) was originally derived under the assumption that s is small, so it is possible that the large s behavior is not accurately captured by (5) and (6).
Similar to Wiehe and Stephan (1993), we define the integral in (6) as Iα,s, but we include the subscript α,s to emphasize that, under some circumstances, Iα,s may be a function of both α and s and cannot be written as a function of only the population-scaled strength of selection. The total rate of coalescence Rc (in coalescent units) due to both sweep and neutral coalescent events is then
| (8) |
The expected height of the coalescent tree for two sequences is the inverse of this rate. The expected reduction in diversity at the neutral locus is proportional to the decrease in the height of the coalescent tree, relative to neutrality:
| (9) |
Wiehe and Stephan (1993) found that Iα,s is approximately constant (I = 0.075) over a range of large values of α:
| (10) |
Note that this removes the dependence on s, which is asserted by (9). In the following sections we show that both (9) and (10) may not hold when s is large.
Materials and Methods
Simulating RHH models
We performed forward simulations of RHH with SFS_CODE (Hernandez 2008) (see Appendix for details). A pictorial representation of the model is shown in Figure 1.
All simulations in this article were performed with θ = 0.002 at the neutral locus, and reductions in diversity were calculated as the ratio of the observed diversity to 0.002, unless otherwise noted. Nucleotide diversity π and Tajima’s D were calculated with a custom script. We often report the difference proportion in (diversity in a rescaled population of size N1) compared to (diversity in the population of size N0), which we define as . For each simulation we sampled 10 individuals (20 chromosomes) from the population. The neutral loci in all simulations are 1 kb in length.
Fixing the probability of fixation
Throughout this article, we discuss appropriate choices of r, λ, s, L, and N for simulations. However, in forward simulations, the rate of substitution is not explicitly provided to the software, but rather a rate of mutation. To calculate the appropriate mutation rate for a simulation, one must incorporate the probability of fixation for a positively selected site. For s < 0.1, the fixation probability of Kimura (1962) is sufficient:
| (11) |
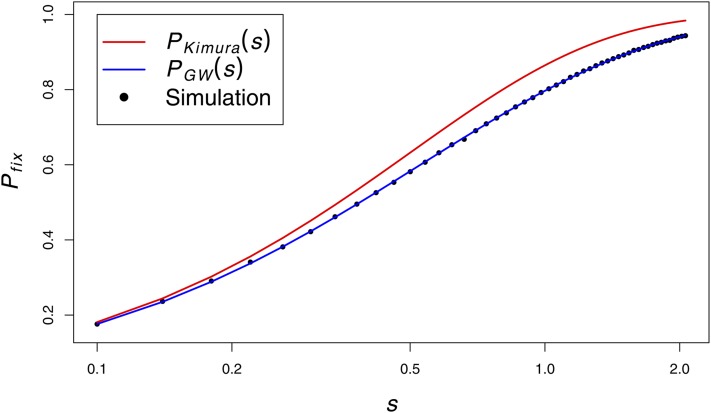
However, when s > 0.1, this approximation overestimates the probability of fixation. For s > 0.1, we treat the initial trajectory of the selected site as a Galton–Watson process and calculate the probability of extinction by generation i, Pe(i), with procedure PGW(s) (Fisher 1999):
procedure PGW(s)
Pe(0) = e−(1+s)
i = 1
while Pe(i) − Pe(i − 1) > δ do
Pe(i) = e−(1+s)(1−Pe(i−1))
i ← i + 1
end while
return 1 − Pe(i)
end procedure
In practice, this algorithm takes <200 iterations to converge for s > 0.1 and provides accurate results (Figure 2). Simulations in Figure 2 were performed with a simple Wright–Fisher simulator that sampled only the trajectory of the selected site and followed it until either (1) loss or (2) the frequency of the selected site exceeded , which very nearly guarantees eventual fixation.
Figure 2.
The probability of fixation (Pfix) as a function of s. Simulation points represent the fraction of fixations in 105 simulations. N = 104.
All analysis and simulation scripts used in this article are available upon request from the authors.
Results
A naive approach to parameter rescaling
In forward simulations, the most computationally costly parameters are N and L, so we seek to reduce these parameters as much as possible. A simple and widely used rescaling assumption is that patterns of diversity are conserved when population-scaled parameters ρ = 4Nr, α = 2Ns, and θ = 4Nμ are held fixed and N is varied (Kim and Wiehe 2009) (for more discussion, see section 5 of the SFS_CODE manual). This is equivalent to the statement that the effective population size is not a fundamental parameter of the dynamics and is similar to the rescaling strategy described in Hoggart et al. (2007), which was not designed specifically for RHH simulations.
Equation 9 provides an informed view of rescaling that incorporates RHH theory. Equation 9 predicts that the impact of the underlying genealogical process on neutral sequence depends on the compound parameters Ns and , but not directly on ρ. Hence, if s is increased and N decreased while holding their product constant, and r and λ are increased while holding their ratio constant, (9) predicts that patterns of variation will be maintained.
Finally, (3) suggests as we decrease N and increase s for fixed α we must also increase the length of the flanking sequence, because selection at more distant sites can affect the neutral locus as s is increased. Note that we can accomplish this either by fixing the recombination rate and increasing the length in base pairs of the flanking region or by increasing the recombination rate for some fixed flanking length. Since the same number of mutations is introduced, these options are functionally identical, but the latter may require less RAM for some forward simulation implementations.
Taken together, these scaling principles suggest a simple algorithm for choosing simulated values of N1, α1, r1, and λ1 that are conservative with respect to the genealogical process as predicted by (9). We wish to model L0 flanking base pairs of sequence in a population of size N0 with parameters ρ0, α0, and λ0. We choose L1 and N1 to be any computationally convenient flanking length and population size. We compute the remaining simulation parameters with Algorithm 1:
procedure Algorithm 1(ρ0, α0, L0, λ0, N0, N1, L1)
Let ; ;
α1 = α0
ρ1 = 4N1r1
return N1, ρ1, α1, L1, λ1
end procedure
Note that if we choose L1 = L0, we obtain α1 = α0, ρ1 = ρ0, and 4N1λ1 = 4N0λ0, which is consistent with the rescaling strategy of Hoggart et al. (2007) and diffusion theory.
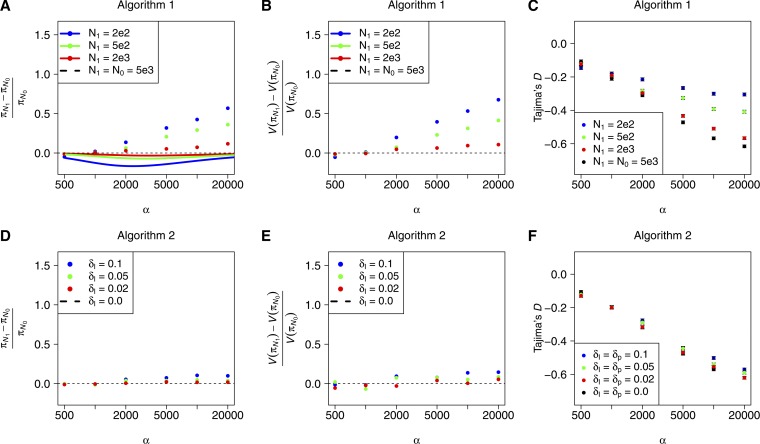
In Figure 3, A–C, we show results obtained with Algorithm 1. In Figure 3A, we plot the normalized difference in mean diversity between simulations performed in a population with N0 = 5000 and simulations performed with rescaled parameters and varying choices of N1. The dashed black line at 0 represents the expectation under perfect rescaling, because perfect rescaling will result in a normalized difference in means equal to 0 between rescaled parameters and the original parameters. The colored circles each represent the mean of 5000 simulations and the solid colored curves were explicitly calculated with (9).
Figure 3.
Mean and variance of observed diversity in the rescaled populations (N1) relative to the model population (N0 = 5000). Rescaled parameters were obtained with Algorithm 1 in A, B, and C and with Algorithm 2 in D, E, and F. A total of 10,000 simulations were performed for each parameter combination. The theoretical curves in A were calculated with (9) in Mathematica (Wolfram Research 2010). Parameters: N0 = 5 × 103, ρ0 = 10−3, λ0 = 10−10, L0 = 106, L1 = 105. C and F show the mean Tajima’s D for the same simulations. Error bars in C and F are the standard error of the mean.
Algorithm 1 generates patterns of diversity in the rescaled populations (colored circles, Figure 3A) that are similar to the simulated diversity in the model population (black dashed line, Figure 3A) when the strength of selection is low, but the algorithm performs poorly when the strength of selection gets arbitrarily large. Qualitatively similar results are observed for the variance in π (Figure 3B) and Tajima’s D (Figure 3C). Furthermore, the mean diversity of simulations performed with Algorithm 1 does not agree well with explicit calculation of the expected diversity using (9) when selection is strong, as seen by the divergence between the mean diversity in the simulations and the solid curves. In fact, (9) predicts that the diversity will decrease as N grows because of the dependence of Iα,s on s (Figure 3A, solid colored curves), but simulations show the opposite pattern. This demonstrates that the simulated value of s has some effect on the expected patterns of diversity (which is not predicted by the results of Wiehe and Stephan 1993, which we used to build Algorithm 1) and that (9) does not appropriately model this dependence.
In the next sections we examine circumstances under which the assumptions used to derive (9) and (10) may break down, and we use insights from this analysis to design a more robust approach to parameter rescaling.
RHH with large values of s
We have predicated Algorithm 1 on (9) and (10), and hence it is likely that it will not perform adequately in parameter regimes in which (9) or (10) is not accurate. Equation 9 was derived using (3), which used the assumption that s is small, so it is possible that in the large s regime (9) will fail to accurately predict the reduction in diversity. Here, we derive a theoretical form that describes the impact of RHH in the large s regime by conditioning on the altered dynamics of the selected locus under very strong selection.
For genic selection, the dynamics of the selected locus are described by
| (12) |
For small s, the denominator of (12) is very close to 1 and is typically ignored [and was ignored in the derivation of (3) by Stephan et al. 1992]. However, for very large s the denominator is nonnegligible, which slows the rate of growth of the selected site when it is at moderate to high frequency. To investigate RHH with large s, we solved (2) approximately, conditioning on (12) for the dynamics of the selected site (see Appendix for derivation). We find
| (13) |
This result differs by only a factor of from (3), but makes very different predictions for large s and rf. As s increases, more distant sites can affect the diversity at the neutral locus. In fact, s can be made arbitrarily large whereas rf is constrained to remain <0.5. As a result, we expect that (9) will underestimate the observed diversity for large s. If we use Algorithm 1 to make N arbitrarily small and s arbitrarily large, (13) predicts that patterns of diversity in the simulated population may be significantly different from the larger population because of this s dependence. We denote the reduction in diversity calculated with (13) as
| (14) |
with an asterisk to differentiate it from (9). As before, is computed as the integral of (13).
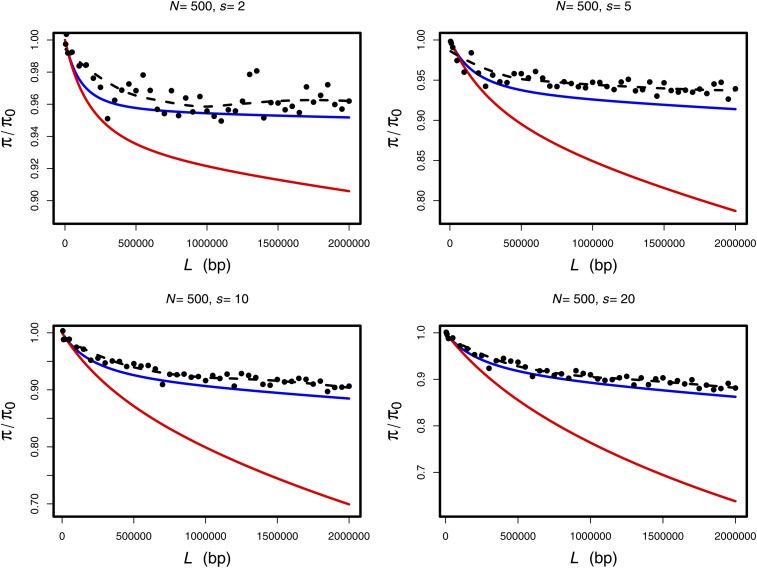
We performed simulations of RHH with large values of s to test (14). We find that (14) accurately predicts the impact of RHH on diversity for large s, whereas (9) is a poor predictor in the large s regime (Figure 4). We have performed this analysis primarily to explain the biased patterns of diversity produced by Algorithm 1, but we note that in some cases (e.g., microbes under extreme selection pressures), it is possible that s can be >>0.1. Indeed, one experimental evolution study of Pseudomonas fluorescens reported values of s as large as 5 and a mean value of 2.1 (Barrett et al. 2006). If and when s achieves such large values in recombining organisms, it will be advantageous to use Equation 13 in place of (3).
Figure 4.
Simulations of recurrent hitchhiking with differing amounts of flanking sequence. In each panel, we vary the amount of flanking sequence and calculate the expected reduction in diversity using (9) (red curves) or (14) (blue curves), where we have used as the upper bound of the integration for calculating Iα,s and . Simulation points (black circles) each represent the mean of 5000 simulations, and the dashed black lines represent loess smoothing of the simulated data.
Robust parameter rescaling for RHH simulations
Using the results in the previous section, we modify Algorithm 1 to guard against violating the assumptions of the RHH model as we rescale the parameters of the simulations.
Let N1, L1, etc., be defined as in the previous section. Our goal is to reduce N0 as much as possible without altering the underlying dynamical process by more than a prespecified amount. Let δI be the maximum deviation between for a population of size N1 and the model population of size N0 that we are willing to accept in our simulations. For example, let δI = 0.01 if we desire simulated sequences in which in a population of size N1 differs by no more than 1% from a population of size N0. Let δp be the maximum difference between in populations of size N0 and N1 that we are willing to accept in our simulations, over all u from 0 to u∗, the length of the flanking region. Qualitatively, δI is a constraint on the total area under , which influences the overall level of diversity, while δp is a constraint on the shape of , which influences the coalescent dynamics for a substitution that occurs at a given distance from the neutral locus. See Figure 5 for a pictorial explanation. We formalize these constraints in Algorithm 2. Note that every parameter chosen by Algorithm 2 is exactly consistent with Algorithm 1. The only difference is that we precompute how small we can make N without altering the dynamics of the RHH model:
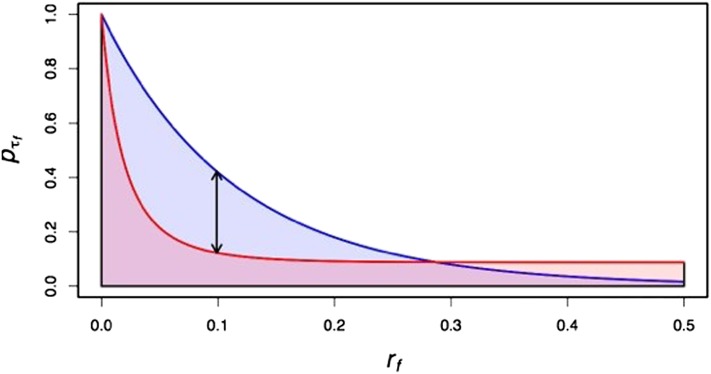
Figure 5.
Algorithm 2 computes the difference between in the population that we wish to model (blue) and a simulated population of smaller size (red) and bounds the difference in the probability of identity in the simulated and model populations. δI represents the maximum difference between the area under the red curve and the area under the blue curve that is acceptable in simulations. δp represents the maximum difference between the red and blue curves that is acceptable over all values of rf (represented by the black double-headed arrow). Qualitatively, δI constrains the overall diversity in the simulated sequences, while δp constrains the shape of the probability of common ancestry during a sweep as a function of recombination distance.
procedure Algorithm 2 (ρ0, α0, L0, N0, λ0, L1, δI, δp)
Let ; ; ;
α1 = α0 = α
Numerically solve for the quantity s1.
over all u on [0, umax]
if D > δp, then numerically solve for s1 over all u on [0, umax]
end if
ρ1 = 4 N1r1
return N1, ρ1, α1, L1, λ1
end procedure
We implemented Algorithm 2 in Python, using the numerical optimization tools in SciPy (Jones et al. 2001) for the numerical optimization steps. In Figure 3, D–F, we demonstrate the performance of Algorithm 2 for three different values of δI = δp. Smaller values of δ generate sequences that are more closely matched to the diversity in the population of size N0, but require a larger simulated N1 and are hence more computationally intensive. Note that for the values of δ that we have chosen herein, only a very small change in the overall diversity is expected. While larger values of δ may be acceptable for some applications, we do not recommend large values in general because the underlying dynamics are not necessarily expected to be conserved even if the change in overall diversity is small. Indeed, small deviations in mean π and Tajima’s D are observed for the largest value of δ with strong selection (Figure 3F).
Computational performance of the rescaled simulations is shown in Figure A3 (see Appendix).
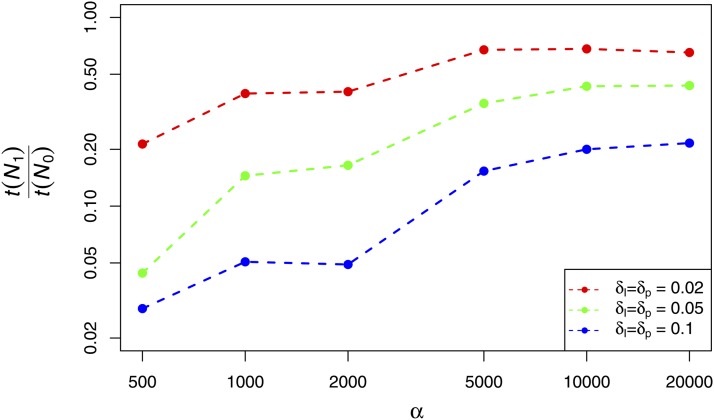
Figure A3.
Duration of rescaled simulations (t(N1)) relative to nonscaled simulations (t(N0)). Parameters were chosen to match Figure 3. Parameters: N0 = 5 × 103, ρ0 = 10−3, λ0 = 10−10, L0 = 106, L1 = 105.
The notion of “sufficiently distant” flanking sites
We designed Algorithm 2 to work for any given L0 in a population of size N0. In the RHH literature, flanking regions of are of particular interest because Equation 3 suggests that sites that are bp from the neutral locus have no impact on the neutral site (Jensen et al. 2008), at least for small s. However, this result does not hold in the large s regime. First, the recombination fraction is not linear in the number of base pairs of flanking sequence when rf > 0.1, and rf cannot exceed 0.5, even for arbitrarily long flanking sequences. Equations 3 and 13 are functions of , so as s gets large (s > 0.5) it is not possible to make compensatory linear increases in rf. Second, the dynamics of the selected site are altered when s is large, as we noted in the previous sections. In particular, (13) suggests that sites that have rf = 0.5 (unlinked sites) have a nonnegligible impact on the diversity at the neutral site when s is very large. The model predicts the impact of Lu unlinked sites to be
| (15) |
Figure 6 shows the reduction in diversity, relative to neutrality, for simulations of RHH that include a neutral region and Lu unlinked selected sites and no linked selected sites. Equation 15 accurately predicts the reduction in diversity for these simulations. These results highlight another problem with Algorithm 1. In Algorithm 1, we linearly increase the flanking sequence as s increases. However, for large s, the majority of these flanking sites are essentially unlinked to the neutral locus, but can have a nonnegligible impact on the neutral locus. This is fundamentally different from the dynamics in the small s regime, where unlinked sites have no impact on the neutral locus.
Figure 6.
Simulations of RHH including only a neutral region and Lu explicitly unlinked selected sites. Lu = 2000, λ = 0.5 × 10−6. Solid circles represent the mean of 4 × 104 simulations, and solid lines were calculated with Equation 15 in Mathematica.
While this result may not be intuitive, it is a natural consequence of very large values of s. Consider the implausible but instructive case when s ≈ 2N. In the first generation after the selected site is introduced into the population, approximately half of the offspring are expected to be descendants of the individual with the selected site. At a locus that is unlinked to the selected site, one of the two chromosomes of the individual with the selected mutant is chosen with equal probability for each of the descendants, which causes an abrupt and marked decrease in diversity. Although this effect is more subtle in our simulations in Figure 6 (which have 1 < s ≪ 2N), there is a measurable decrease in π due to the accumulated effect of unlinked sites with large s.
The role of interference
In the previous sections, we restricted our analysis to parameter regimes in which interference between selected sites is very rare, which is an assumption of the RHH model. However, one of the advantages of forward simulations is that they can be performed under conditions with high levels of interference.
In Figure 7, we examine the performance of Algorithm 2 with very high rates of positive selection. Note that the value of λ on the x-axis is the expected value in the absence of interference in a population of size N0, and the observed value of λ in the simulations is slightly lower. Circle sizes in Figure 7 indicate the amount of interference between selected sites, as measured by the fraction of selected substitutions that overlap with at least one other substitution while segregating in the population. This is a conservative metric for the total effect of interference because it does not include the fraction of selected sites that are lost due to competition with other selected sites.
Figure 7.
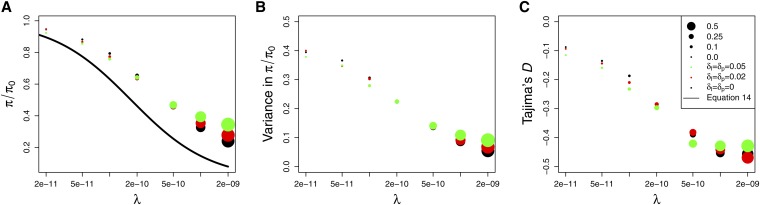
Rescaled simulations of RHH with increasing amounts of interference. A total of 10,000 simulations were performed for each data point (circles). Circle sizes are indicative of the amount of interference in the simulations, as measured by the fraction of selected substitutions that overlap with at least one other positively selected substituted allele while both are segregating in the population. (A) Diversity relative to the neutral expectation, (B) variance in relative diversity, and (C) Tajima's D. Parameters: α = 2 × 103, , r0 = 5 × 10−8, N0 = 5 × 103.
As the rate of interference increases, the theoretical predictions of Equation 13 underestimate the reduction in diversity by an increasing amount (Figure 7A, black circles). This is expected because as interference increases, a smaller fraction of selected sites reach fixation, and furthermore the trajectories of the sites that fix are altered due to competition. Neither of these effects is modeled by Equation 13.
More strikingly, as the rate of interference increases, the separation between the rescaled populations (green and red circles) and the original population (black circles) also increases. This demonstrates that Algorithm 2 does not recapitulate the expected diversity in the rescaled populations when the rate of interference is high in the population of size N0. This result is expected when we consider that the rate of interference is a function of both the rate of substitutions and the time that selected substitutions segregate in the population before fixation. It is well known that the time to fixation is a function of both α = 2Ns and N and cannot be written naturally as a function of only one or the other. Hence, when we rescale the population with fixed α, we necessarily change the amount of interference. We analyze this effect in more detail in the next section when we perform rescaling for two sets of parameters inferred in Drosophila.
An application to Drosophila parameters
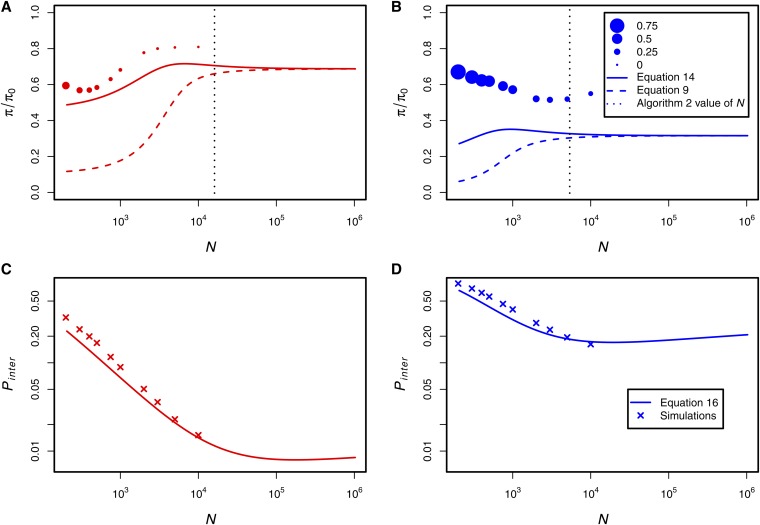
In Figure 8, we perform rescaling with RHH parameters that are relevant to Drosophila. Macpherson et al. (2007) found evidence supporting strong selection (s = 0.01), which occurred relatively infrequently (λ = 3.6 × 10−12) in a Drosophila population of size N0 = 1.5 × 106. Jensen et al. (2008) found weaker (s = 0.002), more frequent selection (λ = 10−10) in a population of N0 = 106. Our goal is not to debate the “true” parameters, but rather to investigate the practicability of rescaling using previously inferred parameters. Assuming a flanking sequence length of and a recombination rate of r0 = 2.5 × 10−8, we apply Algorithms 1 and 2 to these parameter sets and investigate the effect on diversity.
Figure 8.
The reduction in diversity under previously inferred RHH parameters. A and C use the parameters inferred by Macpherson et al. (2007) while B and D use the parameters inferred by Jensen et al. (2008). The solid curves in A and B are given by (14), and the dashed curves are given by (9). C and D show the probability of interference as measured by the proportion of substitutions that are introduced while another substitution is segregating in the population. The solid curves are given by (16). The size of the circles in A and B is proportional to the observed value of pinter, which is plotted in C and D. The dotted vertical lines show the value of N, where δI = δp = 0.05.
In Figure 8A, we show that the trend in simulated diversity (solid red curve) as a function of N under the Macpherson et al. (2007) parameters is correctly described by (14), which predicts that the diversity decreases at low N. However, the model slightly underestimates the mean diversity compared to simulations. In contrast, the model predictions of the diversity are very inaccurate under the parameters estimated by Jensen et al. (2008) (Figure 8B, solid blue curve). In Figure 8, A and B, Algorithm 1 strongly alters the patterns of diversity as N is decreased. The value of N1 calculated with Algorithm 2 and δI = δp = 0.06 are shown with the dotted vertical line.
Circle sizes in Figure 8, A and B, indicate the proportion of substitutions that are introduced while another substitution is on the way to fixation (as in Figure 7). While the interference is fairly mild for large values of N under the parameters of Macpherson et al. (2007), the amount of interference is extreme at all values of N under the Jensen et al. (2008) parameters. In both cases the amount of interference in the simulations changes as we rescale N1.
We designed Algorithm 2 under the assumption that interference is negligible in the population of size N0. This assumption is approximately met under the parameters of Macpherson et al. (2007), where sweeps are infrequent and overlapping sweeps are rare. However, this assumption is broken by the parameters of Jensen et al. (2008), where the rate of sweeps is more than an order of magnitude higher. As a result, the diversity is not well predicted by Equation 14 at any value of N, and Algorithm 2 fails to generate sequences with accurate patterns of genetic diversity.
In general, it is useful to know a priori when the assumptions of the RHH model are not met as a result of high interference in a population of size N0. Consider the probability that a positively selected substitution arises in the population while another substitution is heading toward fixation, pinter. Under the assumption that interference is sufficiently infrequent such that the mean time to fixation and probability of fixation are not strongly altered, we can approximate pinter as
| (16) |
The probability of no substitution in a single generation is 1 − 2Lλ, and hence the probability that no new selected substitutions are introduced while a given selected mutation is on its way to fixation is . Supposing that and plugging in the expectation of τf garner the rest of the terms in Equation 16. The final approximation is valid for very small . Note that we do not expect (16) to hold exactly in any parameter regime because the time to fixation is actually a random variable (and furthermore, both τf and λ are altered when interference is frequent), but we find that pinter is a useful approximation for describing the interference in simulations during the rescaling process.
In Figure 8, C and D, we investigate the behavior of (16) as we rescale the population size. As N is decreased from N0, the value of pinter initially decreases because the product (1 + s)log 2N decreases. However, as N gets very small with Algorithm 1, (1 + s)log 2N eventually begins to increase because 1 + s increases faster than log 2N decreases, increasing the amount of interference.
Although the exact calculation of the effects of interference is very challenging, it is straightforward to calculate the value of pinter in a population of size N0. If the value of pinter is large (e.g., >0.05, as with the parameters in Figure 8B), then the assumptions of Algorithm 2 are broken and there is no guarantee that the rescaled simulated sequences will be sufficiently accurate. By contrast, if there is low interference in a population of size N0, then it is safe to perform rescaling as long as the value of pinter is constrained. In practice, the value of pinter (or other quantities that are related to the rate of interference) can be taken as an additional constraint in the calculation of N1 in Algorithm 2 such that the impact of interference is limited under rescaling.
Discussion
Simulations are an integral part of population genetics because it is often difficult to obtain exact analytical expressions for many quantities of interest, such as likelihoods for sequence data under a given model. Until recently, forward simulations were not practical because of the large computational burden that they can impose. However, several new forward simulation techniques have been proposed and published (Hoggart et al. 2007; Hernandez 2008; Zanini and Neher 2012; Aberer and Stamatakis 2013; Messer 2013), and their use in population genetic studies is becoming increasingly popular.
Despite these computational advances, it remains very computationally intensive to simulate large populations and long chromosomes in a forward context. It is frequently necessary to perform parameter rescaling to achieve computational feasibility for parameter regimes of interest (e.g., N > 105 with long flanking sequences), particularly for applications such as approximate Bayesian computation that require millions of simulations for accurate inference. The hope of such rescaling efforts is that expected patterns of diversity will be maintained after rescaling and that the underlying genealogical process will remain unaltered.
In this investigation, we tested a naive approach to parameter rescaling and showed that this approach can strongly alter the expected patterns of diversity because it does not conserve the underlying genealogical process at the neutral site. In particular, for fixed values of α, s can get arbitrarily large as N is decreased, and previous theoretical results do not accurately predict the patterns of diversity in this parameter regime. We derived a new theoretical form for the reduction in diversity when s is large and show that it has strong predictive power in simulations. We leveraged this result to develop a simple rescaling scheme (Algorithm 2) that approximately conserves the underlying genealogical process. We note that in practice Algorithm 2 may not always be necessary, and as long as s remains small (say, <0.1), Algorithm 1 will suffice. The advantage of Algorithm 2 is that it allows us to quantitate the effect of rescaling and to get the best possible computational performance for a given error tolerance.
It will be of great interest to extend the rescaling results for recurrent sweeps presented herein to models that include arbitrary changes in population size and interference between selected sites. In the case of changes in population size, we note that the strategy presented in Algorithm 2 can be easily extended to perform optimization across a range of population sizes such that the constraints are simultaneously satisfied at all time points in a simulation. This strategy is consistent with previous approaches to rescaling in the context of complex demography (Hoggart et al. 2007).
Interference poses a greater challenge, because the amount of interference is dependent both on the rate of substitution and on the time that selected sites segregate. It is well known that the time to fixation of selected alleles cannot be written as a simple function of α and depends on N as well, and hence rescaling population size with fixed α alters the effects of interference on sequence diversity (Comeron and Kreitman 2002). Improved understanding of scaling laws for interference may be necessary to develop appropriate rescaling strategies, to the extent that such rescaling is possible at all in a forward simulation context. However, recent progress was made in the case of very strong interference, where scaling laws were recently derived by Weissman and Barton (2012).
The rescaling results presented here pose an interesting dilemma for the use of forward simulations in population genetic studies, as previously noted by Kim and Wiehe (2009). A major appeal of forward simulations (compared to coalescent simulators) is the ability to incorporate arbitrary models (e.g., interference between selected sites, complex demographic processes) without knowing anything about the distribution of sample paths a priori. However, if simulations are feasible only when the parameters are rescaled, there is no guarantee for any given theoretical model that the rescaling will maintain expected dynamics. We also note that the rescaling method proposed herein was informed by in-depth knowledge provided by the previous work of several authors and that in general it may not always be obvious which parameters must be simultaneously adjusted to maintain expected patterns of variation in simulations for a given complex model.
Nonetheless, forward simulation in population genetics has a bright future. Forward simulation remains the only way to simulate arbitrarily complex models. For many populations of interest (e.g., ancestral human populations), population size is sufficiently small such that it can often be directly simulated without rescaling. Continued computational advances in both hardware and software in coming years will expand the boundaries of computational performance of forward simulation. Finally, active development of the theory of positive selection, interfering selected sites, background selection, demographic processes, and the joint action thereof will lend further insight into parameter rescaling and advances in the use of forward simulations in population genetic studies.
Acknowledgments
We thank John Pool for a stimulating discussion that motivated this research and Zachary A. Szpiech, Raul Torres, M. Cyrus Maher, Kevin Thornton, Joachim Hermisson, and two anonymous reviewers for comments on the manuscript. This work was partially supported by the National Institutes of Health (grants P60MD006902, UL1RR024131, 1R21HG007233, 1R21CA178706, and 1R01HL117004-01) and a Sloan Foundation Research Fellowship (to R.D.H.), by an Achievement Rewards for College Scientists Foundation Fellowship (to L.H.U.), and by National Institutes of Health training grant T32GM008155 (to L.H.U.'s graduate program).
Appendix
Derivation of for Large s
In this section, we solve for the probability of identity when the selection coefficient is large. We are concerned with the probability of identity p at various frequencies throughout the sweep process. We subscript p with t(x) to indicate the value of p at the time when the selected site reaches frequency x (e.g., ). The trajectory of the selected site for large s is given in (12), while the dynamics of the neutral site are given by (2). We transform (2) into allele frequency space by dividing by (12). We obtain
| (A1) |
This equation can be solved in Mathematica with the initial condition , meaning that all backgrounds carrying the selected locus are identical at the neutral locus when the selected site is introduced. At the end of the sweep, x ≈ 1. We take the solution with because x = 1 results in a singularity:
| (A2) |
Equation A2 can be numerically integrated in Mathematica. However, this solution is complicated, is slow to evaluate, and provides little intuition about the dynamics. As an alternative, we employ an approximate solution strategy.
Following Barton (1998) and others, we subdivide the trajectory of the selected allele into low-frequency and high-frequency portions. For small x, the term (1 + 2sx) ≈ 1, even for large s. As a result, there is little difference between the dynamics for small s and large s sweeps at low frequency. We rewrite (A1) as
| (A3) |
which is valid for low x. We define the solution to (A3) on the interval as pt(ϵ).
For x > ϵ, the second term on the RHS of (A1) dominates the first term, because the first term is inversely proportional to the number of selected chromosomes. To obtain the high-frequency dynamics of the selected allele, we take pt(ϵ) as the initial condition and solve the following differential equation on the interval x = [ϵ, 1]:
| (A4) |
We find the solution
| (A5) |
We can perform the exact same analysis under the assumption that the dynamics are given by , as was done by Stephan et al. (1992) (SWL in Equation A6). This garners the solution
| (A6) |
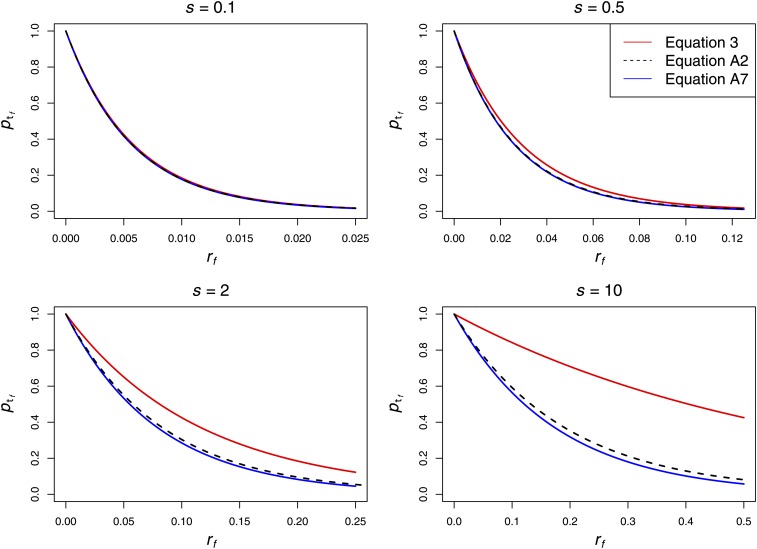
which differs by only a factor of from (A5). Since (A6) was derived under assumptions identical to those used in Stephan et al. (1992), we conclude that sweeps with large s can be modeled with the equation
| (A7) |
This equation provides very similar results to (3) for small s, as expected, but deviates for large s (Figure A1).
Figure A1.
Equation A7 (blue) and Equation A2 (black) compared to Equation 3 (red). As expected, (A7) and (3) are in very close agreement for small s, but diverge for large s. Equation A7 is a good approximation to (A2) across a wide range of values of s. α = 104.
To verify that this approximation provides accurate results to the full solution given by Equation A2, we compared (A2) to (A7) in Mathematica. Agreement is very good between the exact and approximate solutions for all values of s that we investigated (Figure A1).
RHH Simulations in SFS_CODE
We performed forward simulations of RHH with SFS_CODE (Hernandez 2008). An example command line for a RHH simulation is as follows:
sfs_code 1 10 -t <θ> -Z -r <ρ> -N <N> -n <n> -L 3 <L> <l0> <L> -a N R -v L A 1 -v L 1 <Rmid> -W L 0 1 <α> 1 0 -W L 2 1 <α> 1 0
All of these options are described in the SFS_CODE manual, which is freely available online at sfscode.sourceforge.net or by request from the authors. Briefly, this command line runs 10 simulations of a single population of size N and samples n individuals at the end. The recombination rate is set to ρ, and three loci are included in the simulation. The middle locus (locus 1) is l0 bp long while the flanking loci are L bp long. The middle locus is neutral, while the flanking loci contain selected sites with selection strength α = 2Ns. Every mutation in the flanking region is positively selected. The sequence is set to be noncoding with the option “-a N r”. The “-v” option provides the flexibility to designate different rates of mutation at different loci, and the mechanics of its usage are described in detail in the SFS_CODE manual. Rmid specifies the rate at which mutations are introduced into the middle segment relative to the flanking sequences. Please see the SFS_CODE manual for a detailed example of parameter choice for RHH simulations.
Forward simulations of DNA sequences can require large amounts of RAM and many computations. Recurrent hitchhiking models are particularly challenging to simulate because very long sequences must be simulated. In particular, for a given selection coefficient s, RHH theory suggests that sites as distant as rf ≈ s must be included in the simulation to include all sufficiently distant sites (see Equation 3).
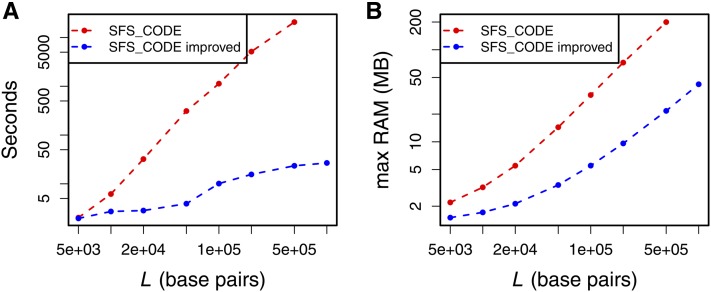
In many organisms, r ≈ 10−8. Assuming rf ≈ rL, this implies that a selection coefficient of s = 0.1 would require 107 bp of simulated sequence on each side of the neutral locus to include all possible impactful sites. This is a prohibitively large amount of sequence for many reasonably chosen values of θ and N in forward simulations (Figure A2). However, in simulations of RHH, we are primarily interested in examining the diversity at a short, neutral locus. We adapted SFS_CODE such that individual loci can have different mutation rates and different proportions of selected sites. For RHH simulations, we set the proportion of selected sites to 0 in the neutral locus and 1 in the flanking sequence. This greatly increases the speed and decreases RAM requirements for SFS_CODE because much less genetic diversity is generated in the flanking sequences (Figure A2, blue curves). Time and RAM usage were measured with the Unix utility “time” with the command “/usr/bin/time -f ‘%e %M’ sfs_code [options]”. Note that time reports a maximum resident set size that is too large by a factor of 4 due to an error in unit conversion on some platforms, which we have corrected herein. Simulations were performed on the QB3 cluster at University of California, San Francisco, which contains nodes with a variety of architectures and differing amounts of computational load at any given time. As such, the estimates of efficiency herein should be taken only as qualitative observations.
Figure A2.
The computational burden of simulations of recurrent positive selection in SFS_CODE is much lower in the new version of SFS_CODE. Both RAM requirements and time to complete the simulations are reduced. Points represent the mean of 500 simulations. θ = 10−3, ρ = 10−3, N = 500, λ = 10−9, α = 1000.
Efficiency of Rescaled Simulations
We report the time to completion of rescaled simulations relative to nonscaled populations, using Algorithm 2 (Figure A3). We observe reductions in time between ∼99% and 40% for the parameters under consideration here. In general, the best performance is obtained for weaker selection, since in this case s is small in the population of size N0, meaning that the value of N can be changed quite dramatically without breaking the small s approximation. Better gains are also observed as the error threshold is increased, but this comes at an accuracy cost (see Robust parameter scaling for RHH simulations).
Footnotes
Communicating editor: J. Hermisson
Literature Cited
- Aberer A. J., Stamatakis A., 2013. Rapid forward-in-time simulation at the chromosome and genome level. BMC Bioinformatics 14: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbiza L., Gronau I., Aksoy B. A., Hubisz M. J., Gulko B., et al. , 2013. Genome-wide inference of natural selection on human transcription factor binding sites. Nat. Genet. 45: 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog D., 2008. Similar rates of protein adaptation in Drosophila miranda and D. melanogaster, two species with different current effective population sizes. BMC Evol. Biol. 8: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett R. D., MacLean R. C., Bell G., 2006. Mutations of intermediate effect are responsible for adaptation in evolving Pseudomonas fluorescens populations. Biol. Lett. 2: 236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N. H., 1998. The effect of hitch-hiking on neutral genealogies. Genet. Res. 72: 123–133. [Google Scholar]
- Beaumont M. A., Zhang W., Balding D. J., 2002. Approximate Bayesian computation in population genetics. Genetics 162: 2025–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante C. D., Fledel-Alon A., Williamson S., Nielsen R., Hubisz M. T., et al. , 2005. Natural selection on protein-coding genes in the human genome. Nature 437: 1153–1157. [DOI] [PubMed] [Google Scholar]
- Chevin L.-M., Billiard S., Hospital F., 2008. Hitchhiking both ways: effect of two interfering selective sweeps on linked neutral variation. Genetics 180: 301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeron J. M., Kreitman M., 2002. Population, evolutionary and genomic consequences of interference selection. Genetics 161: 389–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coop G., Ralph P., 2012. Patterns of neutral diversity under general models of selective sweeps. Genetics 192: 205–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisci J. L., Poh Y.-P., Bean A., Simkin A., Jensen J. D., 2012. Recent progress in polymorphism-based population genetic inference. J. Hered. 103: 287–296. [DOI] [PubMed] [Google Scholar]
- Cutter A. D., Payseur B. A., 2013. Genomic signatures of selection at linked sites: unifying the disparity among species. Nat. Rev. Genet. 14: 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. A., 1999 The Genetical Theory of Natural Selection: A Complete Variorum Edition. Oxford University Press, London/New York/Oxford. [Google Scholar]
- Haldane J., 1919. The combination of linkage values and the calculation of distances between the loci of linked factors. J. Genet. 8: 299–309. [Google Scholar]
- Hernandez R. D., 2008. A flexible forward simulator for populations subject to selection and demography. Bioinformatics 24: 2786–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggart C. J., Chadeau-Hyam M., Clark T. G., Lampariello R., Whittaker J. C., et al. , 2007. Sequence-level population simulations over large genomic regions. Genetics 177: 1725–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson P. K., 2010. Natural selection on synonymous and nonsynonymous mutations shapes patterns of polymorphism in Populus tremula. Mol. Biol. Evol. 27: 650–660. [DOI] [PubMed] [Google Scholar]
- Jensen J. D., Thornton K. R., Andolfatto P., 2008. An approximate Bayesian estimator suggests strong, recurrent selective sweeps in Drosophila. PLoS Genet. 4: e100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E., T. Oliphant, P. Peterson, 2001 SciPy: Open source scientific tools for Python. Available at: http://www.scipy.org/scipylib/citing.html
- Kaplan N. L., Hudson R. R., Langley C. H., 1989. The “hitchhiking effect” revisited. Genetics 123: 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Stephan W., 2003. Selective sweeps in the presence of interference among partially linked loci. Genetics 164: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Wiehe T., 2009. Simulation of DNA sequence evolution under models of recent directional selection. Brief. Bioinform. 10: 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., 1962. On the probability of fixation of mutant genes in a population. Genetics 47: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson J. M., Sella G., Davis J. C., Petrov D. A., 2007. Genomewide spatial correspondence between nonsynonymous divergence and neutral polymorphism reveals extensive adaptation in Drosophila. Genetics 177: 2083–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher M. C., Uricchio L. H., Torgerson D. G., Hernandez R. D., 2012. Population genetics of rare variants and complex diseases. Hum. Hered. 74: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer P. W., 2013. Slim: simulating evolution with selection and linkage. Genetics 194: 1037–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T., Kimura M., 1975. The effect of selected linked locus on heterozygosity of neutral alleles (the hitch-hiking effect). Genet. Res. 25: 313–326. [DOI] [PubMed] [Google Scholar]
- Pool J. E., Hellmann I., Jensen J. D., Nielsen R., 2010. Population genetic inference from genomic sequence variation. Genome Res. 20: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. D., Jensen J. D., Clark A. G., Aquadro C. F., 2013. Inferences of demography and selection in an African population of Drosophila melanogaster. Genetics 193: 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Haigh J., 1974. The hitch-hiking effect of a favourable gene. Genet. Res. 23: 23–35. [PubMed] [Google Scholar]
- Stephan W., Wiehe T. H., Lenz M. W., 1992. The effect of strongly selected substitutions on neutral polymorphism: analytical results based on diffusion theory. Theor. Popul. Biol. 41: 237–254. [Google Scholar]
- Stephan W., Song Y. S., Langley C. H., 2006. The hitchhiking effect on linkage disequilibrium between linked neutral loci. Genetics 172: 2647–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson D. G., Boyko A. R., Hernandez R. D., Indap A., Hu X., et al. , 2009. Evolutionary processes acting on candidate cis-regulatory regions in humans inferred from patterns of polymorphism and divergence. PLoS Genet. 5: e1000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D. B., Barton N. H., 2012. Limits to the rate of adaptive substitution in sexual populations. PLoS Genet. 8: e1002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiehe T. H., Stephan W., 1993. Analysis of a genetic hitchhiking model, and its application to DNA polymorphism data from Drosophila melanogaster. Mol. Biol. Evol. 10: 842–854. [DOI] [PubMed] [Google Scholar]
- Wolfram Research, 2010 Mathematica: Version 8.0. Wolfram Research, Champaign, IL.
- Zanini F., Neher R. A., 2012. Ffpopsim: an efficient forward simulation package for the evolution of large populations. Bioinformatics 28: 3332–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]