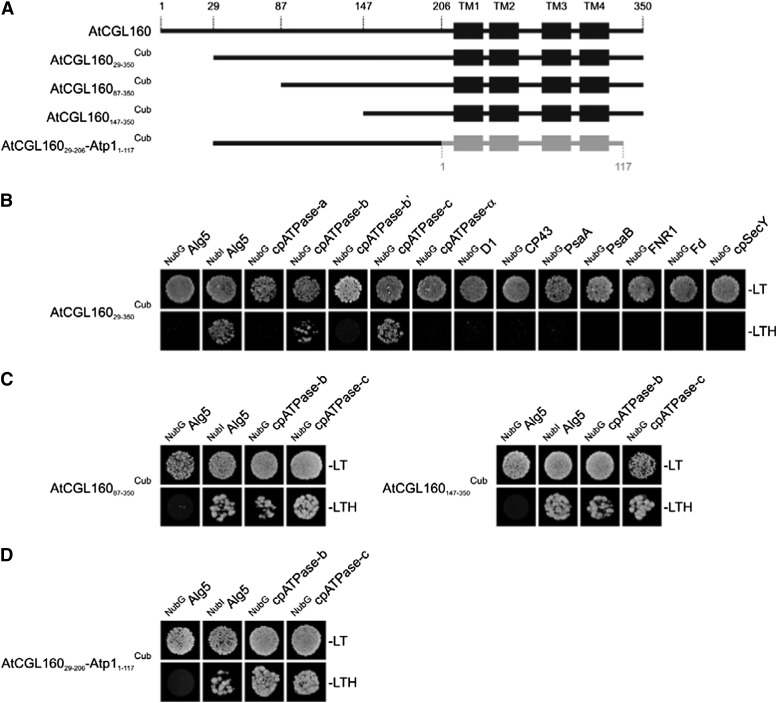
Figure 9.
Split-ubiquitin assays for interactions between AtCGL160 and selected thylakoid proteins. A, Schematic depictions of the four constructs used in the tests presented in B to D. TM domains of AtCGL160 and Atp1 are schematically shown as black and gray boxes, respectively, and amino acid positions are indicated above the full-length sequence of AtCGL160. B, Split-ubiquitin assays with mature AtCGL160 (AtCGL16029-350Cub). Assays were performed using fusions to the C-terminal (Cub) and N-terminal (NubG) halves of ubiquitin. NubIAlg5 (the unrelated endoplasmic reticulum membrane protein Alg5 fused to the wild-type Nub) served as a positive control. Alg5 fused to NubG (NubGAlg5) was used as the negative control. To test for interactions involving the AtCGL160 protein, the mature AtCGL160 protein was fused to Cub (AtCGL160Cub) and the selected thylakoid proteins were fused to NubG. Yeast colonies were first plated on permissive medium (−LT, lacking Leu and Trp; top row) and then on selective medium (−LTH, lacking Leu, Trp, and His; bottom row; see “Materials and Methods”). C, Interaction assays with the two N-terminally truncated fragments AtCGL16087-350 and AtCGL160147-350. D, Split-ubiquitin analyses with a fusion construct consisting of the N terminus of AtCGL160 (amino acids 29–206) and the full-length Atp1 (amino acids 1–117) from Synechocystis sp. PCC6803.