Abstract
The goal of this study was to compare the effects of minocycline and simvastatin on functional recovery and brain gene expression after a cortical contusion impact (CCI) injury. Dosage regimens were designed to provide serum concentrations in a rat model in the range obtained with clinically approved doses; minocycline 60 mg/kg q12h and simvastatin 10 mg/kg q12h for 72 h. Functional recovery was assessed using motor and spatial learning tasks and neuropathological measurements. Microarray-based transcriptional profiling was used to determine the effect on gene expression at 24 h, 72 h, and 7 days post-CCI. Gene Ontology analysis (GOA) was used to evaluate the effect on relevant biological pathways. Both minocycline and simvastatin improved fine motor function, but not gross motor or cognitive function. Minocycline modestly decreased lesion size with no effect of simvastatin. At 24 h post-CCI, GOA identified a significant effect of minocycline on chemotaxis, blood circulation, immune response, and cell to cell signaling pathways. Inflammatory pathways were affected by minocycline only at the 72 h time point. There was a minimal effect of simvastatin on gene expression 24 h after injury, with increasing effects at 72 h and 7 days. GOA identified a significant effect of simvastatin on inflammatory response at 72 h and 7 days. In conclusion, treatment with minocycline and simvastatin resulted in significant effects on gene expression in the brain reflecting adequate brain penetration without producing significant neurorestorative effects.
Key words: : cortical contusion injury model, gene expression, minocycline, simvastatin, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a serious problem facing the medical community. In the United States, 1.7 million TBIs occur annually.1 Despite many years of research, there are currently no approved pharmacotherapies for the treatment of patients with TBI. This may in part be because of the complicated secondary cascade of damage that occurs after the initial impact. The etiology of the secondary cascade resulting from TBI is likely from interrelated processes including mitochondrial energy failure, excessive generation of reactive oxygen species, activation of destructive enzymes, membrane disruption, neuronal death, thrombosis from intravascular coagulation in small vessels, increased synaptic concentrations of excitatory amino acids, and activation of innate inflammatory responses.2–4
The inflammatory response to TBI has been noted as being considerably up-regulated, even months after injury.5 In addition, many researchers have noted that the inflammatory response likely plays a dual-role in TBI, assisting in mitigating immediate consequences of injury, but that extended action likely contributes to detrimental consequences.6 Preventing the inflammatory response is considered a high potential target for neuroprotection after TBI.3,7 Two such agents, minocycline and simvastatin, have come to the attention of the field and represent particularly interesting targets because they are already clinically approved for other uses.
Minocycline is a lipid-soluble antibiotic. There have been more than 300 publications demonstrating neuroprotection properties in experimental models of ischemic injury, Parkinson disease, Huntington disease, Alzheimer disease, multiple sclerosis, amyotrophic lateral sclerosis (for review, see Plane and associates8), and TBI.9–17 Minocycline has been shown to have anti-inflammatory, anti-apoptotic, and anti-oxidant properties.8 Simvastatin is a member of the HMG-reductase inhibitors, a group of anti-cholesterol drugs, many of which have been found to have anti-inflammatory properties.18 Simvastatin has been found to be as beneficial or, in some cases, more beneficial than atorvastatin, for the management of experimental TBI.19,20 In a cortical contusion injury (CCI) rodent model, simvastatin at a dose of 1 mg/kg/day selectively down-regulated the pro-inflammatory cytokine, interleukin-1β (Il-1β) 72 h post-CCI, while not reducing the secretion of anti-inflammatory cytokine, interleukin-6,21 which some data suggest is neuroprotective.22 Simvastatin also may reduce apoptosis by means of Akt-mediated pathways.23
The objective of the current study was to characterize the effects of minocycline and simvastatin on changes in gene expression and behavioral function after a unilateral TBI. This study is part of an on-going project that assesses and compares the effectiveness of several drugs on TBI. The goal is to identify the most beneficial treatment and also identify the mechanism of action using microarray analysis. The ultimate goal is to use these data to design a multi-drug treatment therapy that will also be tested in our TBI model. Before the CCI studies, pharmacokinetic studies were performed in uninjured animals to determine the dose regimens needed to provide average serum concentrations in the range of the concentrations obtained clinically with Food and Drug Administration (FDA) approved doses.
Methods
Animals and housing
Male Sprague-Dawley rats, obtained from Harlan Laboratories (Indianapolis, IN), approximately 3 months old with mean body weight of 350 g, were used in this study. For the pharmacokinetic studies, the rats were obtained with surgically implanted jugular vein catheters. All animal and surgical procedures were adhered to as described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Southern Illinois University Institutional Animal Care and Use Committee (IACUC) and the University of Washington's IACUC reviewed and approved all experimental procedures. Animals were housed in a university-maintained, Association for Assessment and Accreditation of Laboratory Animal Care accredited vivarium, with a 12-h light/dark schedule and a controlled environmental temperature of 22°C in standard housing cages with food and water available ad libitum.
Pharmacokinetic studies
Based on initial single dose studies, minocycline 60 mg/kg (n=4) and simvastatin 10 mg/kg (n=4), obtained from Sigma-Aldrich, St. Louis, were administered by oral gavage every 12 h for 72 h. Minocycline is a highly sclerosing agent24 and has been shown to cause tissue damage, scarring, and inflammation if administered by intraperitoneal (i.p.) injection.25 Simvastatin is a lactone pro-drug (inactive itself), which is hydrolyzed to its active metabolite simvastatin acid and other metabolites in the intestinal wall and liver. Blood specimens were collected from the jugular catheter, at 0, 1, 2, 4, 6, 11.5, 23.5, 26, 47.5, 50, 71.5, and 74 h for the minocycline study and 0, 1, 2, 4, 6, 14, 26, 38, 50, 62, and 74 h for the simvastatin study. Samples were collected in microtubes, separated using a centrifuge, and stored at −80o C until assayed. The terminal exponential rate constant () was used to determine elimination half-life (T1/2) as 0.693/.
Drug assays
Minocycline was analyzed in serum using high pressure liquid chromatograph with UV detection at 352 nm on a Varian Pro Star 210 HPLC system. Hydrochloride salts of minocycline and the internal standard demeclocycline were obtained from Sigma-Aldrich (St. Louis). Serum samples (40 μL) containing the internal standard and an equal volume of 0.1M sodium phosphate were adjusted to a pH of 2.05, deproteinized with 1 mL methanol, and centrifuged. The supernate was dried under nitrogen at 40–50°C and reconstituted with 100 uL 0.1M sodium phosphate buffer, pH 2.05. Samples were injected onto a Sunfire C-18, 3.5 μm, 150×4.6 mm column kept at 40°C (Waters, Milford, MA) and separated using a mobile phase gradient of 10–22% acetonitrile in 0.1M sodium phosphate at pH 2.05 over 4 min, held at 22% for 3 min, then returned to 10% until end of the run at 16.5 min. Minocycline concentrations were targeted at 5–20 μg/mL.26
Because simvastatin is a pro-drug with active metabolites, HMG-CoA activity in plasma was estimated using a HMG-CoA Reductase Assay Kit (Sigma-Aldrich, St. Louis) adapted for quantitation of enzyme inhibition by pravastatin. Calibration standards ranged from 10–200 ng/mL of pravastatin in a 0.03125M K2HPO4:0.75 % KCl: 0.1% bovine serum albumin buffer adjusted to pH 6.22 with o-phosphoric acid. Aliquots of reconstituted kit components and calibration standards were stored at −80°C and used only once after thawed and kept on ice. Plasma samples to be analyzed were deproteinized with acetonitrile and centrifuged at 4°C. The supernate was evaporated under N2 and reconstituted in the buffer used for the calibration standards.
Sixty μL of blank buffer, calibrators, or plasma samples, 29.5 μL kit assay buffer, 2 μL reconstituted NADPH, 6 μL HMG-CoA, and 2.5 μL of HMG-CoA reductase solution diluted in 82 μL of kit assay buffer were added to 96 well microplates equipped with half area wells (Corning Inc., Corning). Plates were immediately placed in a Benchmark microplate reader (BioRad Laboratories) set at 37°C and incubated for 5 min. After mixing for 1 min, using the kinetic mode, readings were taken every 20 sec (with 10 sec of mixing before every read) at 340 nm to monitor the reduction in absorbance from the oxidation of NADPH.
Average velocity over the measuring interval from 2.5–23.3 min was computed for each well. A linear regression equation was computed using log pravastatin concentration versus average velocity for the calibration standards. The average velocity measured for the rat plasma samples was used to calculate as pravastatin equivalents by regression analysis. Simvastatin concentrations were targeted based on pravastatin equivalents. A 19.2 mg oral dose of pravastatin, a dose within the therapeutic dosing range, resulted in average peak concentrations of 27±11 ng/mL in a group of healthy subjects.27
Surgery
Aseptic surgery was performed according to previous protocols.28,29 Animals were anesthetized with a combination of isofluorane (2–4%) and oxygen (0.8L/min), and body temperature was maintained at 37°C. A midline incision was made through the skin and underlying fascia. A circular craniotomy (5.0 mm) was centered 2.4 mm posterior to and 2.4 mm lateral (left) to bregma. A 4.0 mm stainless steel impactor tip, attached to an electromagnetic impactor (myneurolab.com), was used to induce the CCI injury. The cortex was impacted at 3.0 m/sec to a depth of 2.5 mm for 0.5 sec. After the injury was induced, the incision was sutured closed, and the animal was allowed to recover in a heated chamber until locomotor function returned.
Functional behavioral studies
Rats were randomly assigned to four groups. Doses of minocycline (60 mg/kg), simvastatin (10 mg/kg), or vehicle (0.9% saline, 4.8 mL/kg), were administered via oral gavage beginning at 2 h post-surgery and continued every 12 h until 72 h post-surgery. Group one received CCI and was administered minocycline (n=8). Group two received CCI and was administered simvastatin (n=7). Group three received CCI and was administered vehicle (n=8). Group four received sham procedures and was administered vehicle (n=8). Sham animals received an “intact sham” procedure according to previous protocols 30 in which they were anesthetized, a midline incision was made, sutured closed, and then they were placed in the recovery chamber.
Locomotor placing task
To assess fine motor function, rats were tested on the locomotor placing task according to previous protocols.29,31 Rats were pretested on the day before surgery to assess baseline performance. Rats were placed on an elevated grid surface (56×54 cm) with openings measuring 3.2×3.2 cm and allowed to freely explore. The number of grid lines crossed was recorded (movement) as were the number of “foot faults” when an animal placed a limb through one of the openings. The following equation was used to calculate foot faults on the impaired limb as a function of lines crossed: ([Right Faults–Left Faults]/Lines Crossed). One trial of 180 sec was given on days 7, 9, 11, 14, and 16 post-surgery.
Rotarod task
To evaluate gross locomotor dysfunction after brain injury, rats were tested on the rotarod according to a previous protocol.32 A 7 cm diameter cylinder was positioned 1.2 m above a foam pad while speed and acceleration were controlled by computer interface (San Diego Instruments, San Diego, CA). Pre-training occurred on the 2 days preceding injury. Initially, an adaptation trial was performed in which rats were familiarized with the consequences of falling by repeatedly replacing them on the cylinder while it was stationary. Once they were able to stay on for 1 min, training began. A constant acceleration paradigm was used (0.055 cm/sec2), and the latency to fall was recorded. Four trials were given per day with an intertrial of approximately 10 min. After injury, rats were retested on the rotarod task on days 7–11 post-surgery.
Morris water maze (MWM)
To determine the extent of cognitive deficits, rats were tested on the MWM according to a previous protocol.29 Two phases of testing were conducted: reference memory and working memory. The reference memory paradigm testing occurred on days 15–18 post-surgery. A clear Plexiglas platform (10×10 cm) was submerged in 32 cm of room temperature water in the center of the northeast quadrant of a 1.5 m diameter pool. Rats were lowered from a random start position into the water facing the wall of the tank. A trial ended when 90 sec had elapsed or the rat had reached the platform. Rats unable to complete the task in 90 sec were guided by hand to the platform. Rats were allowed to remain on the platform for 10 sec and then removed and placed in a heated cage. There were four trials per day with a 15 min intertrial interval. The latency and distance to reach the platform was recorded for each trial.
The working memory paradigm was assessed on days 21–23 post-surgery. The procedure was the same as above, except the position of the platform was changed on each test day (northwest, southwest and southeast quadrant). There were four trials per day with a 15 min inter-trial interval. The first trial was considered to be an acquisition trial and not analyzed.
Lesion analysis
At day 25 post-surgery, rats were given a lethal dose of a sodium pentobarbital solution (Euthasol, Virbac Animal Health; 0.3 mL, i.p.). Once eye-blink and pedal responses disappeared, rats were transcardially perfused with ice-cold 0.9% phosphate-buffered saline, followed by 10% phosphate-buffered formalin. Brains were then coronally sliced, frozen, on a sliding microtome at 40 μm and mounted to gelatin-subbed slides. Five coronal sections transversing the lesion were selected (−0.4, −1.4, −2.4, −3.4, −4.4) and stained with cresyl-violet. An Olympus microscope (BX-51) with an Olympus 13.5 megapixel camera (DP-70) was used to image the sections. The area of each hemisphere was measured using ImageJ software (NIH, Bethesda, MD). A percent reduction score was then calculated by dividing the left (injured) hemisphere by the right (intact) hemisphere.28,29,32,33 These scores were then averaged together to calculate the extent of the injury.
Statistical analyses
All data were analyzed without knowledge of group assignment. The mean and standard error of the mean were calculated for all the data. The effects of treatment on behavioral outcome for each task were analyzed in repeated measures analysis of variance (ANOVA). The Huynh-Feldt correction was used to correct for repeated measures. A one-way ANOVA was used to evaluate the effect of treatment on lesion size. The Fischer least significant difference (LSD) test was used for planned post-hoc comparisons between the vehicle and minocycline groups and the vehicle and simvastatin groups. A p value of less than 0.05 was considered to be statistically significant.
Gene expression studies
Rats were randomly assigned to four groups. Doses of minocycline (60 mg/kg), simvastatin (10 mg/kg), or vehicle (0.9% saline, 4.8 mL/kg), were administered via oral gavage beginning at 2 h post-surgery and continued every 12 h until 72 h post-surgery or sacrifice. Group one received CCI and was administered minocycline (n=15). Group two received CCI and was administered simvastatin (n=15). Group three received CCI and was administered vehicle (n=15). Group four did not receive surgery and was administered vehicle (sham, n=5). Animals were sacrificed and tissues collected at 24 h, 72 h, and 7 days after injury according to previously published protocols.33
At each time point, five animals from each treatment group were sacrificed. Control animals were sacrificed at 72 h so that they received an equivalent amount of experience with gavage vehicle administration to account for any effects related to the stress response. Animals were put under deep anesthesia with a mixture of CO2 (80%) and O2 (20%), a cardiac blood sample taken, and then decapitated. Brains were then rapidly extracted, sliced into a 4 mm coronal slab, placed on a cold plate, and a 5 mm tissue punch taken, which included the injured cortex and a small amount of peri-injury cortex. Tissue samples were then placed in centrifuge tubes, snap frozen, and stored at −80° C. Cardiac blood samples were collected at the time of sacrifice and from the tail vein at 72 h in the 7 day group to verify serum drug levels. Blood serum was separated by microcentrifuge, snap frozen and stored at −80° C.
All samples were shipped by overnight carrier to the University of Washington on dry ice. Minocycline concentrations were assayed as described above. Simvastatin activity was not determined in the CCI studies because of problems with stability of HMG-reductase activity during transport.
The processing and analysis of the RNA samples, the microarray analysis using the AffymetrixGeneChip® 3000 scanner, and Bioconductor package p.adjust34 were performed as described previously.30,31 We performed Gene Ontology category analysis via the cumulative hypergeometric distribution method to determine enhanced Gene Ontology categories35 using the Bioconductor package GOstats.36 We used differentially expressed genes (more than 1.5 fold up or down regulated, p<0.05) for this analysis to identify Gene Ontology categories by evidence of overrepresentation of significant genes. The validation of the data obtained with the microarrays was performed using fluorogenic 5' -nuclease-based assay and quantitative reverse transcription-polymerase chain reaction (RT-PCR) as previously described.30,31 The RT-PCR data were normalized to the housekeeping genes, -actin and glyceraldehyde 3-phosphate dehydrogenase (GADPH).
Results
Pharmacokinetics
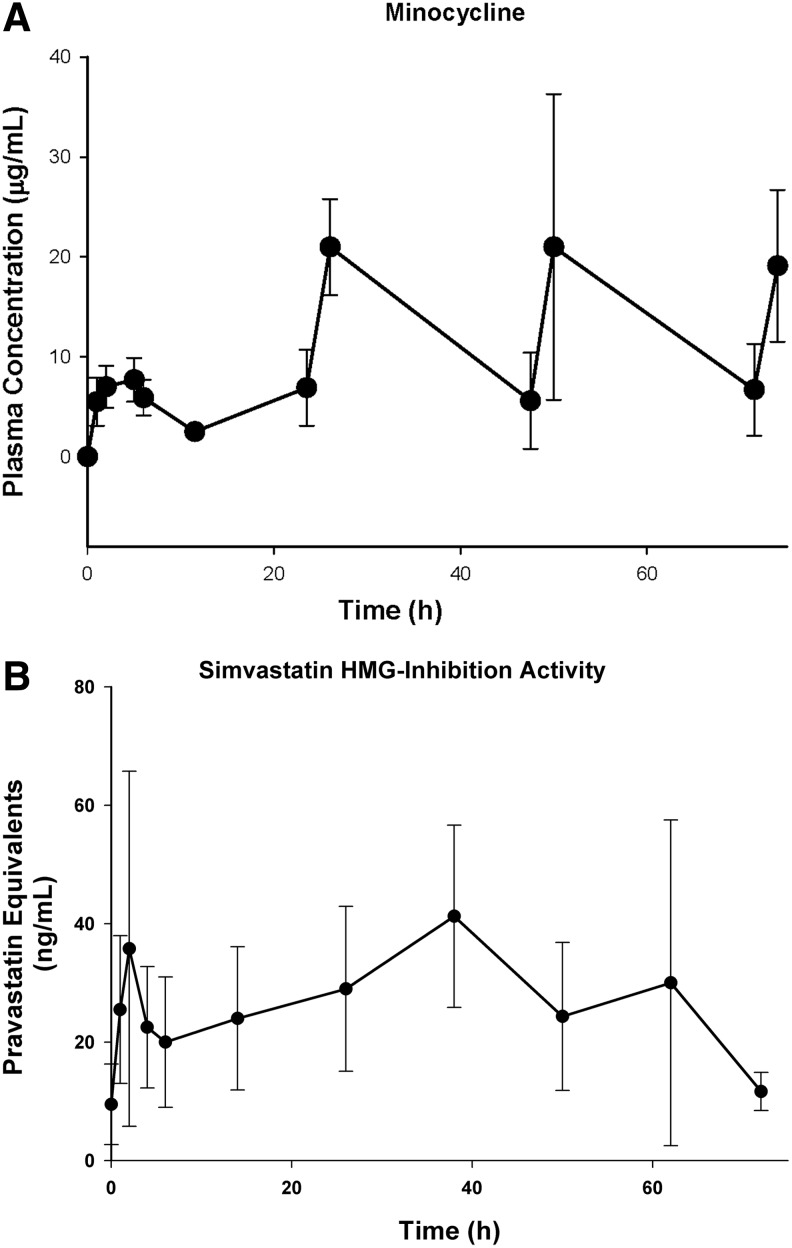
In the non-injured animals, minocycline peak concentrations ranged from 5.5–10.5 μg/mL, with an average T1/2 of 4.6 h after the first oral dose (Fig. 1A). Subsequent doses resulted in average peak concentrations of 21±5 μg/mL, 21±15 μg/mL, and 19±8 μg/mL when sampled 2 h after the 24 h, 48 h, and 72 h doses, respectively, demonstrating a prolonged variable absorption and accumulation. In the gene expression studies, minocycline concentrations were 8±6 μg/mL, and 18±10 μg/mL at 24 h and 72 h time points, consistent with the findings in the non-injured animals. In the functional behavioral study, minocycline concentrations were 17±11 μg/mL after the last minocycline dose at 72 h. Average peak simvastatin concentrations activity measured as pravastatin equivalents were 31±7 ng/mL in the non-injured animals (Fig. 1B) and were within the range of the concentrations attained with a therapeutic dose of pravastatin.27
FIG. 1.
Concentration-time curves. (A) Minocycline 60 mg/kg oral every 12 h for 72 h. (B) Simvastatin 10 mg/kg orally every 12 h for 72 h. After 12 h, only peak concentrations were obtained. Simvastatin activity was determined as pravastatin equivalents.
Functional behavioral study
Locomotor placing
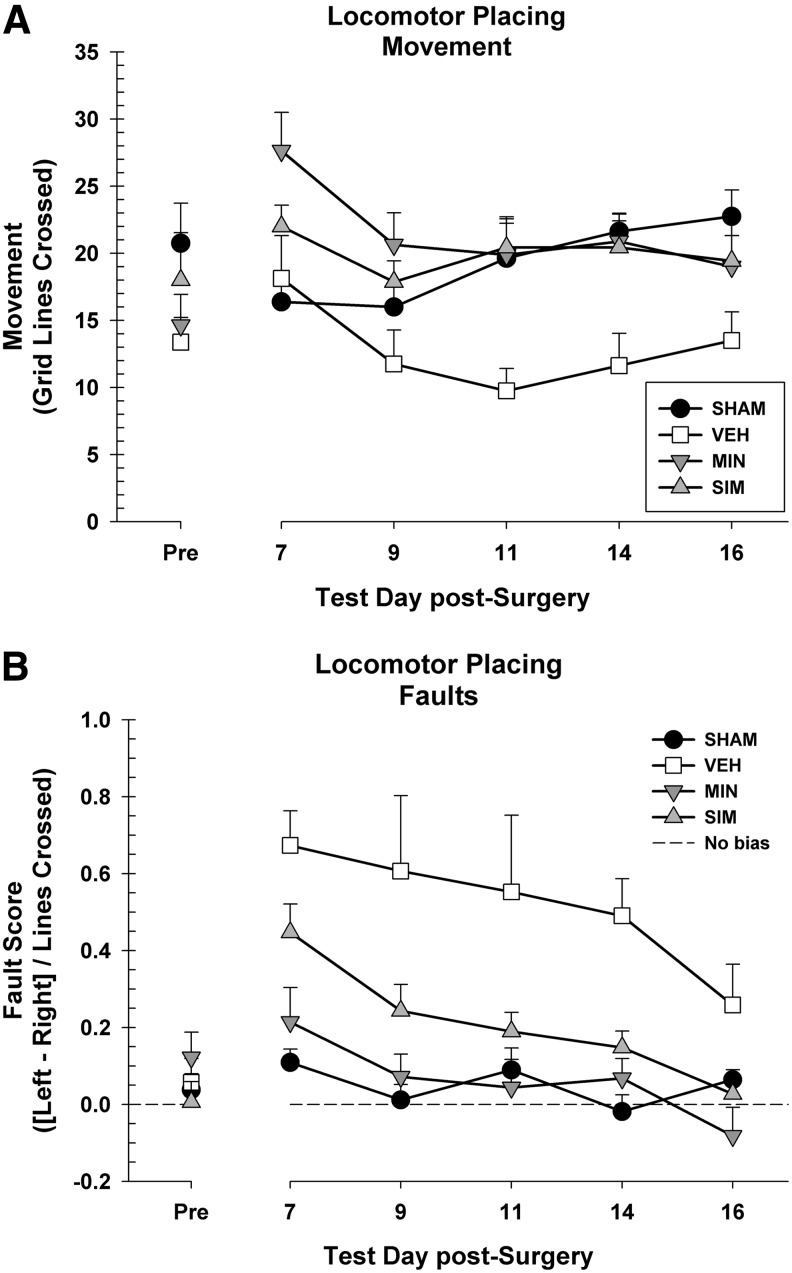
Before injury, there were no differences between the groups in terms of movement, F(3, 27)=1.59, p=0.215, or fault score, F(3, 27)=0.65, p=0.588. Movement on the task post-surgery was analyzed in a 4×5 repeated measures ANOVA (group [sham, vehicle, minocycline, simvastatin]×day [7, 9, 11, 14, 16]) (Fig. 2A). There was a significant main effect of day because of changes in movement across the testing time, F(3.58, 96.74)=5.92, p<0.001. There was a significant difference between the groups, F(3, 27)=3.88, p=0.021. A post-hoc comparison showed that the minocycline group had increased locomotion compared with the vehicle group, LSD (14)=8.65, p=0.004. The simvastatin group also moved more compared with the vehicle group, LSD (13)=7.08, p=0.019. There was also a day×group interaction on movement, F(10.75, 96.74)=3.96, p<0.001, because of the vehicle group decreasing in total movement considerably as testing went on (Fig. 2A).
FIG. 2.
(A) The locomotor placing task showing movement (+standard error of the mean [SEM]) for days 2, 4, 6, 8, and 10 post-cortical contusion impact (CCI). Overall, both the minocycline (MIN p=0.004) and simvastatin (SIM p=0.019) animals moved more than the vehicle (VEH) animals during the task. (B) The locomotor placing task showing the average fault scores (+SEM) for days 2, 4, 6, 8, and 10 post- CCI. Overall, both the minocycline (p<0.001) and simvastatin (p=0.003) animals performed better than the vehicle animals.
The post-surgery fault scores were analyzed in a 4×5 repeated measures ANOVA (group [sham, vehicle, minocycline, simvastatin]×day [7, 9, 11, 14, 16]). There was a significant main effect of day, suggesting that rats improved over time, F(3.36, 90.81)=8.89, p<0.001. There was a significant difference between the groups, F(3, 27)=11.82, p<0.001. Post-hoc tests revealed that minocycline administration improved performance compared with the vehicle group, LSD(14)=0.45, p<0.001. The simvastatin group also showed improved performance relative to the vehicle group, LSD(13)=0.31, p=0.003 (Fig. 2B). There was no day×group interaction on fault score, F(10.09, 90.81)=1.18, p=0.317.
Rotarod
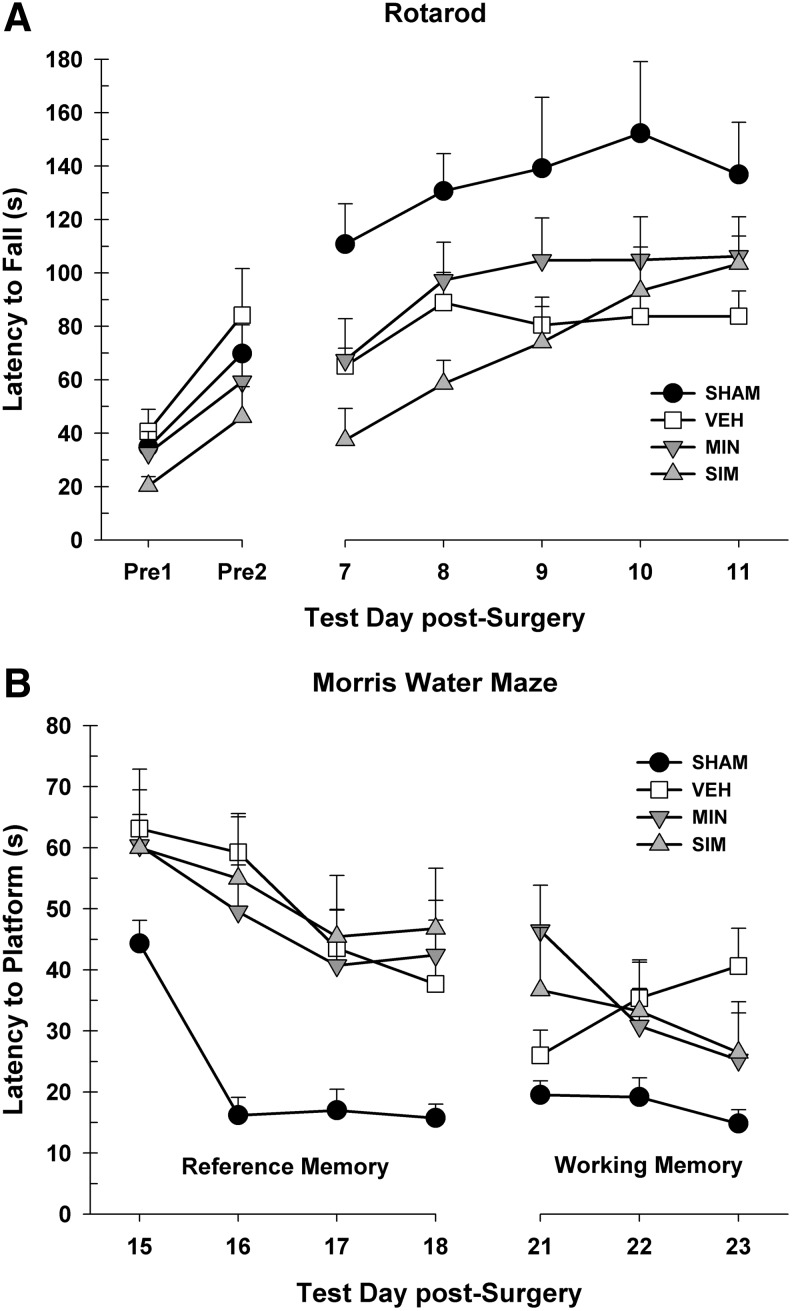
The 2 pre-injury days were averaged together and analyzed in a one-way ANOVA. There were no differences between the groups, F(3, 27)=2.33, p=0.097. Latencies to fall were recorded on the task post-surgery and analyzed in a 4×5 repeated measures ANOVA (group [sham, vehicle, minocycline, simvastatin]×day [7, 8, 9, 10, 11]). There was a significant main effect of day, suggesting that rats improved over time, F(3.59, 96.92)=10.35, p<0.001. There was a significant difference between the groups, F(3, 27)=4.50, p=0.011. There were no significant improvements in the minocycline group, however, compared with the vehicle group, LSD (14)=15.71, p=0.385, or the simvastatin group compared with the vehicle group, LSD(13)=7.06, p=0.704. There was no day×group interaction, F(10.77, 96.92)=1.05, p=0.407 (Fig. 3A).
FIG. 3.
(A) The Rotarod test showing the average latency to fall (+standard error of the mean [SEM]) off of the rotating cylinder for days 8–12 post-cortical contusion impact (CCI). No significant differences were found between the treated animals and vehicle (VEH) animals. (B) The MWM reference memory task showing the average latency (+SEM) to reach the platform on days 14–17 post-CCI. No significant differences were found between the treated animals and VEH animals. The MWM working memory task showing the average latency (+SEM) to reach the platform on days 21–23 post-CCI. No significant differences were found between the treated animals and VEH animals. SIM, simvastatin; MIN, minocycline.
MWM
Latencies to reach the platform were recorded on the reference memory paradigm of the MWM and analyzed in a 4×4 repeated measures ANOVA (group [sham, vehicle, minocycline, simvastatin]×day [15, 16, 17, 18]). There was a significant main effect of day, suggesting that rats improved over time, F(2.99, 80.81)=11.83, p<0.001. There was a significant difference between the groups, F(3, 27)=5.62, p=0.004. Post-hoc tests revealed no differences between the minocycline and vehicle groups, LSD(14)=2.64, p=0.745, or the simvastatin and vehicle groups, LSD(13)=0.89, p=0.915. There was no day×group interaction, F(8.98, 80.81)=0.87, p=0.552 (Fig. 3B).
Latencies to reach the platform were recorded on the working memory paradigm of the MWM and analyzed in a 4×3 repeated measures ANOVA (group [sham, vehicle, minocycline, simvastatin]×day [21, 22, 23]). There was no significant main effect of day, F(2, 54)=1.01, p=0.370. There was no significant difference between the groups, F(3, 27)=2.90, p=0.053 (Fig. 3B). There was no significant day×group interaction, F(6, 54)=2.18, p=0.059. There were also no differences in motor ability (average velocity) in the MWM during the reference memory paradigm, F(3, 27)=0.68, p=0.570, or working memory paradigm, F(3, 27)=1.02, p=0.400.
Lesion analysis
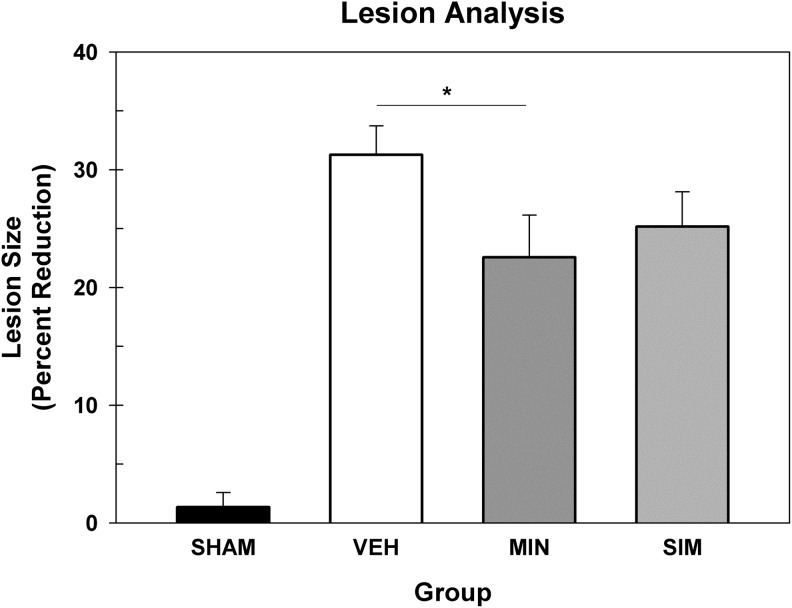
Lesion size (percent reduction of hemisphere) was evaluated in a one-way ANOVA. There was a significant difference between the groups, F(3, 27)=24.06, p<0.001. Post-hoc tests revealed that the minocycline group had smaller lesions compared with the vehicle, LSD(14)=8.69, p=0.028, but the simvastatin group showed no difference, LSD(13)=6.08, p=0.128 (Fig. 4).
FIG. 4.
Lesion analysis. The average (+standard error of the mean) percent reduction score. The minocycline (MIN)-treated group had reduced lesion volumes compared with the vehicle (VEH)-treated group, but the simvastatin (SIM)-treated group showed no improvement. *=p<0.05.
Gene expression study
The microarray data passed all the standard and advanced quality control metrics. The number of differentially expressed genes (>1.5-fold change, p<0.05) at 24 h, 72 h, and 7 days are presented in Table 1. The vehicle to sham comparison reflects the effect of the TBI without treatment relative to sham controls. The minocycline or simvastatin (CCI animals that received treatment) to vehicle (CCI animals that received vehicle) comparison evaluates the effect of treatment on gene expression in the context of TBI.
Table 1.
Total Number of Genes that Were Differentially Expressed (>1.5-Fold up or Down, p<0.05) at 24 h, 72 h, and 7 Days
| 24 h | 72 h | 7 days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Down | Up | Total | Down | Up | Total | Down | Up | Total | |
| Vehicle Sham |
1012 | 1382 | 2394 | 1710 | 2149 | 3859 | 1380 | 1899 | 3279 |
|
Minocycline Vehicle |
103 | 20 | 123 | 71 | 116 | 187 | 55 | 126 | 181 |
|
Simvastatin Vehicle |
5 | 5 | 10 | 67 | 47 | 114 | 30 | 99 | 129 |
Both minocycline and simvastatin treatments resulted in significant changes in brain gene expression in the CCI model demonstrating acceptable brain penetration. At 24 h post-CCI, Gene Ontology analysis (GOA), which shifts the emphasis from evaluation of single genes to evaluation of pathways, networks, and functions, identified a significant effect of minocycline on chemotaxis, blood circulation, immune response, and a variety of cell to cell signaling pathways (Table 2). Inflammatory pathways were only affected by minocycline at the 72 h time point.
Table 2.
Gene Ontology Analysis: Effect of Minocycline Compared with Vehicle
| GO ID | GO Term | Annotated | Significant genes | p value |
|---|---|---|---|---|
| 24 h | ||||
| GO:0006935 | chemotaxis | 284 | Bmp4, C3ar1, Ccr2, Egr2, Trp2,Ntf3,Tgfb2 | 0.00105 |
| GO:0008015 | blood circulation | 297 | C3ar1, Htr2c, Kng1l1, Nts, Scn4b, Sult1a1, Tgfb2 | 0.00136 |
| GO:0050801 | ion homeostatis | 582 | C3ar1, Egr2, Htr2c, Ntf3, Pkp2, Scn4b, Slc9a2, Slc9a4, Tgfb2 | 0.00140 |
| GO:0006584 | catecholamine metabolic process | 40 | Htr2c, Sult1a1, Tgfb2 | 0.00146 |
| GO:0007420 | brain development | 487 | Aldh1a2, Bmp4, Egr2, Gabra5, Neurod1, Neurod6, Ntf3, Tgfb2 | 0.00154 |
| GO:0045055 | regulated secretory pathway | 42 | Btk, Ccr2, Steap2 | 0.00168 |
| GO:0042129 | regulation of T cell proliferation | 94 | Bmp4, Ccr2, Cd3e, RT1-Ba | 0.00180 |
| GO:0008285 | negative regulation of cell proliferation | 407 | Aldh1a2, Bmp4, Ifi30, Ogn, RT1-Ba, Rxfp2, Tgfb2, Xdh | 0.00195 |
| GO:0015812 | GABA transport | 12 | Htr2c, Trpc4 | 0.00190 |
| GO:0021542 | dentate gyrus development | 12 | Neurod1, Neurod6 | 0.00190 |
| GO:0042063 | gliogenesis | 165 | Bmp4, Ccr2, Egr2, Ntf3, Tgfb2 | 0.00230 |
| GO:0007210 | serotonin receptor signaling pathway | 13 | Htr1f, Htr2c | 0.00235 |
| GO:0019886 | antigen processing and presentation of exogenous peptide antigen via MHC class II | 13 | Ifi30, RT1-Ba | 0.00235 |
| GO:0045601 | regulation of endothelial cell differentiation | 13 | Bmp4, Xdh | 0.00235 |
| GO:0007184 | SMAD protein import into nucleus | 14 | Bmp4, Tgfb2 | 0.00273 |
| GO:0046641 | positive regulation of alpha-beta T cell proliferation | 14 | Ccr2, Cd3e | 0.00273 |
| GO:0008347 | glial cell migration | 15 | Ccr2, Tgfb2 | 0.00313 |
| GO:0007267 | cell-cell signaling | 661 | Bmp4, Btk, C1ql3, Egr2, Gabra5, Htr2c, Neurod1, Ntf3, Rgs14, Trpc4 | 0.00365 |
| GO:0050678 | regulation of epithelial cell proliferation | 183 | Aldh1a2, Bmp4, Mmp12, Tgfb2, Xdh | 0.00365 |
| 72 h | ||||
| GO:0006953 | acute-phase response | 41 | Ass1, Il1rn, Kng1, Kng1l1, Ptgs2, Reg3a, Reg3b, Reg3g | 5.26e-09 |
| GO:0007218 | neuropeptide signaling pathway | 64 | Calca, Crhr1, Glra1, Grp, Nmb, Npy | 2.72e-06 |
| GO:0002526 | acute inflammatory response | 100 | Ass1, Il1rn, Kng1, Kng1l1, Ptgs2, Reg3a, Reg3b, Reg3g | 2.80e-06 |
| GO:0007204 | elevation of cytosolic calcium ion concentration | 164 | Agtr1a, Calca, Crhr1, Cxcl2, Kng1, Nmb, Npy2r, Tgfb2 | 7.87e-06 |
| GO:0051051 | negative regulation of transport | 270 | Acvr1c, Calca, Crhr1, Il1rn, Nmb, Npy2r, Ostn, Ptgs2, Tgfb2 | 4.38e-05 |
| GO:0008015 | blood circulation | 297 | Agtr1a, Calca, Hcn4, Kng1, Kng1l1, Nppc, Npy, Ptgs2, Sult1a1, Tgfb2 | 9.14e-05 |
| GO:0006874 | cellular calcium ion homeostasis | 235 | Agtr1a, Calca, Crhr1, Cxcl2, Kng1, Nmb, Npy2r, Tgfb2 | 1.04e-04 |
| GO:0030808 | regulation of nucleotide biosynthetic process | 84 | Calca, Crhr1, Nppc, Npy2r, Ostn | 1.75e-04 |
| GO:0045638 | negative regulation of myeloid cell differentiation | 46 | Calca, Lrrc17, Tnfrsf11b, Zbtb16 | 1.88e-04 |
| GO:0007200 | phospholipase C-activating G-protein coupled receptor signaling pathway | 48 | Agtr1a, Calca, Crhr1, Gpr139 | 2.22e-04 |
| GO:0032845 | negative regulation of homeostatic process | 21 | Calca,Tgfb2, Tnfrsf11b | 2.88e-04 |
| GO:0030155 | regulation of cell adhesion | 209 | Calca, Il1rn, Kng1, Npy2r, Plau, Serpini1, Tgfb2 | 3.18e-04 |
| GO:0033555 | multicellular organismal response to stress | 64 | Calca, Crhr1, Gabra5, Npy2r | 6.73e-04 |
| GO:0070555 | response to interleukin-1 | 73 | Cxcl2, Hnmt, Il1rn, Mmp9 | 1.11e-03 |
| GO:0006805 | xenobiotic metabolic process | 34 | Fmo2, Gsta3, Sult1a1 | 1.22e-03 |
| GO:0006584 | catecholamine metabolic process | 40 | Sult1a1, Sult1d1, Tgfb2 | 1.96e-03 |
| 7 days | ||||
| GO:0042573 | retinoic acid metabolic process | 17 | Aldh1a1, Aldha2, Cyp26b1 | 6.60e-05 |
| GO:0042445 | hormone metabolic process | 148 | Aldh1a1, Aldha2, Bmp5, Cyp26b1, Igf2 | 6.66e-04 |
| GO:0046890 | regulation of lipid biosynthetic process | 97 | Bmp5, Igf2, Prox1, Thrsp | 1.14e-03 |
| GO:0021542 | dentate gyrus development | 12 | Neurod6, Prox1 | 1.42e-03 |
| GO:0007210 | serotonin receptor signaling pathway | 13 | Htr1a, Htr5b | 1.68e-03 |
| GO:0050810 | regulation of steroid biosynthetic process | 50 | Bmp5, Igf2, Prox1 | 1.70e-03 |
| GO:0019216 | regulation of lipid metabolic process | 185 | Chrm5, Igf2, Prox1, Thrsp | 1.81e-03 |
| GO:0021766 | hippocampus development | 58 | Neurod6, Prox1 | 2.61e-03 |
| GO:0007155 | cell adhesion | 573 | Cdh1, Cdh7, Col1a1, Cpxm2, Pkp2, Sell,Tbx18, Thbs1, Wisp2 | 5.36e-03 |
| GO:0050920 | regulation of chemotaxis | 86 | Il16, Sell, Sema3a, Thbs1 | 7.89e-03 |
| GO:0050678 | regulation of epithelial cell proliferation | 183 | Aldh1a2, Bmp5, Prox1, Tbx18, Thbs1 | 1.10e-02 |
| GO:0008285 | negative regulation of cell proliferation | 407 | Aldh1a2, Bmp5, Ogn, Prox1, RT1-Bb, Thbs1, Wisp2 | 1.24e-02 |
| GO:0050679 | positive regulation of epithelial cell proliferation | 105 | Bmp5, Prox1, Tbx18 | 1.36e-02 |
| GO:0006821 | chloride transport | 55 | Gabra5, Slc26a7 | 2.81e-02 |
| GO:0016051 | carbohydrate biosynthetic process | 139 | Chst9, Igf2 | 2.83e-02 |
Acvr1c (activin A receptor, type IC), Agtr1a (angiotensin II receptor, type 1a), Aldh1a1 (aldehyde dehydrogenase 1 family, member A1), Aldh1a2 (aldehyde dehydrogenase 1 family, member A2), Ass1 (argininosuccinate synthase 1), Bmp4 (bone morphogenetic protein 4), Bmp5 (bone morphogenetic protein 5), Btk (Bruton agammaglobulinemia tyrosine kinase), Calca (calcitonin-related polypeptide alpha), C1ql3 (complement component 1, q subcomponent-like 3), C3ar1 (complement component 3a receptor 1), Ccr2 (chemokine (C-C motif) receptor 2), Cd3e (CD3 molecule, epsiolon), Cdh1 (cadherin 1), Cdh7 (cadherin 7, type 2), Chrm5 (cholinergic receptor, muscuarinic 5), Chst9 (carbohydrate (N-acetylgalactosamine 4-0) sulfotransferase 9),Cish (cytokine inducible SH2-containing protein), Col1a1 (collagen, type I, alpha 1), Cpxm2 (carboxypeptidase X (M14 family), member 2), Crhr1 (corticotropin releasing hormone receptor 1), Cxcl2 (chemokine (C-X-C motif) ligand 2), Cyp1b1 (cytochrome P450, family 1, subfamily b,polypepetide 1), Cyp27b1 (cytochrome P450, family 27, subfamily b,polypepetide 1) Cyp26b1 (cytochrome P450, family 26, subfamily b,polypepetide 1)Egr2 (early growth response 2), F13a1 (coagulation factor X111, A1 polypeptide), Fmo2 (flavin containing monooxygenase 2), Gabra5 (gamma-aminobutyric acid (GABA) A receptor, alpha 5), GPr139 (G protein-coupled receptor 139), Grp (gastrin releasing peptide), Gsta3 (glutathione S-transferase A3), Hcn4 (hyperpolarization activated cyclic nucleotide-gated potassium channel 4), Hnmt (histamine N-methyltransferase), Hspb1 (heat shock protein 1), Htr1a (5-hydroxytryptamine (serotonin), receptor 1A, G-protein coupled), Htr1f (5-hydroxytryptamine (serotonin), receptor 1F, G-protein coupled), Htr2c (5-hydroxytryptamine (serotonin) receptor 2C, G-protein coupled), Htr5b (5-hydroxytryptamine (serotonin), receptor 5B), Il1rn (interleukin 1 receptor antagonist), Il16 (interleukin 16), Igf2 (insulin-like growth factor 2), Ili30 (interferon gamma inducible protein 30), Kng1 (kiniogen 1),Kng1l1 (kiniogen 1-like 1), Lrrc17 (leucine rich repeat containing 17), Mmp9 (matrix metallopeptidase 9), Mmp12 (matrix metallopeptidase 12), Mustn1 (musculoskeletal, embryonic nuclear protein 1), Neurod1 (neurogenic differentiation 1), Neurod6 (neurogenic differentiation 6), Nmb (neuromedin B), Nppc (natriuretic peptide C), Npy (neuropeptide Y), Npy2r (neuropeptide Y receptor Y2), Nrp2 (neuropilin 2), Ntf3 (neurotrophin 3), Nts (Neurotensin), Ogn (osteoglycin), Ostn (osteocrin), Pkp2 (plakophilin 2), Plau (plasminogen activator, urokinase), Prox1 (prospero homeobox 1), Ptgs2 (prostaglandin-endoperoxide synthase 2), Reg3a (regenerating islet-derived 3 alpha), Reg3b (regenerating islet-derived 3 beta), Reg3g (regenerating islet-derived 3 gamma), Rgs14 (regulator of G-protein signaling 14), RT1-Ba (RT1 class II, locus Ba), RT1-Bb (RT1 class II, locus Bb), Rxfp2 (relaxin/insulin-like family peptide receptor 2), Scn4b (sodium channel, voltage-gated), Sell (selectin L), Sema3a (sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3A), Serpini1 (serpin peptidase inhibitor, clade I, member 1),Slc26a7 (solute carrier family 26, member 7), Steap2 (Steap family member 4), Sulf1 (sulfatase1), Sult1a1 (sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1), Sult1d1 (sulfotransferase family, cytosolic, 1D, member 1),Tbx18 (T-box18), Tgfb2 (transforming growth factor, beta 2), Tgfbi (transforming growth factor, beta induced), Thbs1 (thrombospondin 1), Thrsp (thyroid hormone responsive), Tnfrsf11b (tumor necrosis factor receptor superfamily, member 11b), Trpc4 (transient receptor potential cation channel, subfamily C, member 4), Wisp2 (WNT1 inducible signaling pathway protein 2), Xdh (xanthine dehydrogenase), Zbtb16 (zinc finger and BTB domain containing 16).
GO, gene ontology.
The effect of minocycline treatment (minocycline/vehicle) and TBI (vehicle/sham) on differentially expressed genes of interest and their specific fold changes in expression are presented in Table 3. Of note, the expression of matrix metallopeptidase 8 (Mmp8), Mmp9, Mmp12, chemokine (C-C motif) receptor 2 (Ccr2), and heat shock protein 1 (Hspb1) were significantly increased by TBI and decreased by minocycline. Minocycline also decreased the expression of interleukin 1 receptor antagonist (Ilrn) and increased the expression of interleukin 16, a pro-inflammatory cytokine, at 72 h and 7 d, post-CCI.
Table 3.
Effect of Minocycline and Traumatic Brain Injury (Vehicle/Sham) on Genes of Interest (1.5 Fold Change, p<0.05)
| Minocycline | Vehicle | |||
|---|---|---|---|---|
| Affymetrix ID | Gene symbol | Genes | Vehicle | Sham |
| 24 h | ||||
| 10911380 | Aldh1a2 | aldehyde dehydrogenase 1 family, member A2 | 0.61 | 0.48 |
| 10782891 | Bmp4 | bone morphogenetic protein 4 | 0.63 | n.s. |
| 10939293 | Btk | Bruton agammaglobulinemia tyrosine kinase | 0.66 | 2.33 |
| 10799733 | C1ql3 | complement component 1, q subcomponent-like 3 | 0.65 | n.s. |
| 10914614 | Ccr2 | chemokine (C-C motif) receptor 2 | 0.64 | 6.19 |
| 10916955 | Cd3e | CD3 molecule, epsilon | 0.51 | n.s. |
| 10848165 | Chrm5 | cholinergic receptor, muscarinic 5 | 0.62 | n.s. |
| 10722347 | Gabra5 | gamma-aminobutyric acid (GABA) A receptor, alpha 5 | 0.56 | n.s. |
| 10787491 | Ifi30 | interferon gamma inducible protein 30 | 0.66 | 4.11 |
| 10755135 | Kng1l1 | kininogen 1-like 1 | 0.51 | 3.26 |
| 10907869 | Mmp12 | matrix metallopeptidase 12 | 0.44 | 7.84 |
| 10846685 | Neurod1 | neurogenic differentiation 1 | 0.41 | n.s. |
| 10862698 | Neurod6 | neurogenic differentiation 6 | 0.50 | 0.60 |
| 10923835 | Nrp2 | neuropilin 2 | 0.67 | 2.13 |
| 10902047 | Nts | Neurotensin | 0.18 | n.s. |
| 10865724 | Ntf3 | neurotrophin 3 | 0.63 | n.s. |
| 10797648 | Ogn | osteoglycin | 0.46 | 0.36 |
| 10752576 | Pkp2 | plakophilin 2 | 0.64 | n.s. |
| 10797274 | Rgs14 | regulator of G-protein signaling 14 | 0.65 | n.s. |
| 10768357 | Rgs18 | regulator of G-protein signaling 18 | 0.54 | n.s. |
| 10828351 | RT1-Ba | RT1 class II, locus Ba | 0.63 | 2.81 |
| 10922909 | Slc9a2 | solute carrier family 9 (sodium/hydrogen exchanger), member 2 | 0.44 | n.s. |
| 10922895 | Slc9a4 | solute carrier family 9 (sodium/hydrogen exchanger), member 4 | 0.26 | n.s. |
| 10725782 | Sult1a1 | sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1 | 0.61 | 0.58 |
| 10815352 | Trpc4 | transient receptor potential cation channel, subfamily C, member 4 | 0.66 | n.s. |
| 10882933 | Xdh | xanthine dehydrogenase | 0.63 | 2.75 |
| 72 h | ||||
| 10845416 | Acvr1c | activin A receptor, type IC | 0.59 | n.s. |
| 10798194 | Agtr1a | angiotensin II receptor, type 1a | 0.29 | 4.17 |
| 10835355 | Ass1 | argininosuccinate synthase 1 | 0.64 | 1.94 |
| 10911711 | Bmp5 | bone morphogenetic protein 5 | 0.66 | n.s. |
| 10725051 | Calca | calcitonin-related polypeptide alpha | 0.50 | 9.91 |
| 10912908 | Cish | cytokine inducible SH2-containing protei | 0.57 | 1.97 |
| 10816405 | Crabp2 | cellular retinoic acid binding protein 2 | 0.49 | 3.81 |
| 10775896 | Cxcl2 | chemokine (C-X-C motif) ligand 2 | 2.43 | n.s. |
| 10771649 | Cxcl11 | chemokine (C-X-C motif) ligand 11 | 0.59 | 3.87 |
| 10887947 | Cyp1b1 | cytochrome P450, family 1, subfamily b, polypeptide 1 | 0.49 | 6.77 |
| 10894200 | Cyp4f4 | cytochrome P450, family 4, subfamily f, polypeptide 4 | 1.57 | 0.41 |
| 10794734 | F13a1 | coagulation factor XIII, A1 polypeptide | 2.00 | 1.99 |
| 10718934 | Fcar | IgA Fc receptor | 1.68 | n.s. |
| 10769370 | Fm02 | flavin containing monooxygenase | 1.97 | n.s. |
| 10722347 | Gabra5 | gamma-aminobutyric acid (GABA) A receptor, alpha 5 | 1.57 | 0.33 |
| 10935811 | Gabre | gamma-aminobutyric acid (GABA) A receptor, epsilon | 0.50 | 2.22 |
| 10891679 | Gpr68 | G protein-coupled receptor 68 | 0.66 | 1.62 |
| 10725286 | Gpr139 | G protein-coupled receptor 139 | 1.60 | n.s. |
| 10926958 | Gsta3 | glutathione S-transferase A3 | 1.54 | 0.52 |
| 10843184 | Hnmt | histamine N-methyltransferase | 1.58 | 0.36 |
| 10761128 | Hspb1 | heat shock protein 1 | 0.65 | 14.24 |
| 10834109 | Il1rn | interleukin 1 receptor antagonist | 0.50 | 13.14 |
| 10888131 | Kcng3 | potassium voltage-gated channel, subfamily G, member 3 | 0.59 | 0.58 |
| 10751988 | Kng1 | kininogen 1 | 0.38 | 4.47 |
| 10842239 | Mmp9 | matrix metallopeptidase 9 | 0.60 | 4.54 |
| 10786532 | Mustn1 | musculoskeletal, embryonic nuclear protein 1 | 0.57 | 2.32 |
| 10723220 | Nmb | neuromedin B | 1.60 | n.s. |
| 10929606 | Nppc | natriuretic peptide C | 1.55 | 0.53 |
| 10855506 | Npy | neuropeptide Y | 0.61 | 1.52 |
| 10815655 | Npy2r | neuropeptide Y receptor Y2 | 0.39 | 3.42 |
| 10850783 | Nrsn2 | neurensin 2 | 1.52 | 0.60 |
| 10755008 | Ostn | osteocrin | 0.43 | 1.58 |
| 10782271 | Plau | plasminogen activator, urokinase | 0.58 | 12.43 |
| 10764551 | Ptgs2 | prostaglandin-endoperoxide synthase 2 | 0.43 | 2.60 |
| 10856481 | Reg3a | regenerating islet-derived 3 alpha | 0.45 | 2.55 |
| 10856474 | Reg3b | regenerating islet-derived 3 beta | 0.47 | 2.07 |
| 10863410 | Reg3g | regenerating islet-derived 3 gamma | 0.10 | 34.41 |
| 10827686 | RT1-M6-1 | RT1 class I, locus M6, gene 1 | 0.53 | n.s. |
| 10827691 | RT1-M6-2 | RT1 class I, locus M6, gene 2 | 0.59 | 1.79 |
| 10815962 | Serpini1 | serpin peptidase inhibitor, clade I, member | 1.51 | 0.38 |
| 10874981 | Sulf1 | sulfatase 1 | 0.65 | 1.84 |
| 10725782 | Sult1a1 | sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1 | 2.10 | 0.64 |
| 10771919 | Sult1d1 | sulfotransferase family 1D, member 1 | 1.69 | 0.30 |
| 10770577 | Tgfb2 | transforming growth factor, beta 2 | 0.58 | 1.98 |
| 10797138 | Tgfbi | transforming growth factor, beta induced | 0.64 | 6.93 |
| 10903725 | Tnfrsf11b | tumor necrosis factor receptor superfamily, member 11b | 0.59 | n.s. |
| 10917116 | Zbtb16 | zinc finger and BTB domain containing 16 | 1.94 | 0.56 |
| 7 days | ||||
| 10714323 | Aldh1a1 | aldehyde dehydrogenase 1 family, member A1 | 1.71 | 0.61 |
| 10911380 | Aldh1a2 | aldehyde dehydrogenase 1 family, member A2 | 0.61 | 2.82 |
| 10807542 | Cdh1 | cadherin 1 | 0.60 | n.s. |
| 10763401 | Cdh7 | cadherin 7, type 2 | 0.64 | n.s. |
| 10848165 | Chrm5 | cholinergic receptor, muscarinic 5 | 1.74 | 0.62 |
| 10803315 | Chst9 | carbohydrate (N-acetylgalactosamine 4-0) sulfotransferase 9 | 1.77 | n.s. |
| 10737532 | Col1a1 | collagen, type I, alpha | 0.64 | 3.57 |
| 10863608 | Cyp26b1 | cytochrome P450, family 26, subfamily b, polypeptide 1 | 0.67 | n.s. |
| 10722347 | Gabra5 | gamma-aminobutyric acid (GABA) A receptor, alpha 5 | 1.60 | 0.45 |
| 10812879 | Htr1a | 5-hydroxytryptamine (serotonin) receptor 1A, G protein-coupled | 1.63 | 0.45 |
| 10767186 | Htr5b | 5-hydroxytryptamine (serotonin) receptor 5B | 1.89 | 0.51 |
| 10726999 | Igf2 | insulin-like growth factor 2 | 0.59 | 2.18 |
| 10723351 | Il16 | interleukin 16 | 1.54 | n.s. |
| 10907913 | Mmp8 | matrix metallopeptidase 8 | 0.63 | 14.59 |
| 10862698 | Neurod6 | neurogenic differentiation 6 | 2.10 | n.s. |
| 10752576 | Pkp2 | plakophilin 2 | 1.80 | 0.57 |
| 10770680 | Prox1 | prospero homeobox | 1.53 | n.s. |
| 10831567 | RT1-Bb | RT1 class II, locus Bb | 0.48 | 4.06 |
| 10817053 | S100a3 | S100 calcium binding protein A3 | 1.72 | 2.76 |
| 10765186 | Sell | selectin L | 0.64 | 2.02 |
| 10860481 | Sema3a | sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3A | 0.66 | 0.66 |
| 10875581 | Slc26a7 | solute carrier family 26, member 7 | 0.63 | n.s. |
| 10919175 | Tbx18 | T-box18 | 0.66 | 2.18 |
| 10723720 | Thrsp | thyroid hormone responsive | 1.51 | 0.58 |
| 10842043 | Wisp2 | WNT1 inducible signaling pathway protein 2 | 0.60 | 1.88 |
There was minimal effect of simvastatin on gene expression 24 h after injury, with increasing effects at 72 h and 7 days post- CCI (Table 1). GOA identified a significant effect of simvastatin on inflammatory response and cell chemotaxis at both the 72 h and 7 day time points (Table 4). The effect of simvastatin treatment (simvastatin/vehicle) and TBI (vehicle/sham) on differentially expressed genes of interest and their specific fold changes in expression are presented in Table 5. Of note, the primary effect of simvastatin on the chemokines, selectin, interleukin 18 (Il-18), Il1rn, S100 binding proteins, and other genes was to increase the expression of genes that were also increased by TBI itself. As has been reported by others,21 Il-1 was increased by TBI 3.7 fold, 2.2 fold, and 1.7 fold at 24 h, 72 h, and 7 days, respectively. There was no effect, however, of simvastatin on Il-1 expression at any time point measured.
Table 4.
Gene Ontology Analysis: Effect of Simvastatin Compared with Vehicle
| GO ID | GO term | Annotated | Significant genes | p value |
|---|---|---|---|---|
| 72 h | ||||
| GO:0006954 | inflammatory response | 341 | Ace, Adcyap1, Agtr1a, Ccl3, Ccl7, Ccl12, Ccl24, Ccr41, Ccr2, Cxcl2, Fabp4, Olr1, Ptgs2, Reg3b, S100a9, Tac1 | 1.05e-11 |
| GO:0030595 | leukocyte chemotaxis | 93 | Ccl3, Ccl7, Ccl12, Ccl24, Ccr1, Ccr2, Cxcl2, S100a9, Sell, Tgfb2 | 3.76e-11 |
| GO:0050729 | positive regulation of inflammatory response | 56 | Ace, Ccl3, Ccl24, Ccr2, Fabp4, Ptgs2, Tac1 | 1.35e-08 |
| GO:0030335 | positive regulation of cell migration | 202 | Ccl3, Ccl7, Ccl24, Ccr1, Ccr2, Fgf1, Ptgs2, Sell, Tac1, Tgfb2 | 7.51e-08 |
| GO:0050727 | regulation of inflammatory response | 156 | Ace, Adcyap1, Ccl3, Ccl24, Ccr1, Ccr2, Fabp4, Ptgs2, Tac1 | 9.71e-08 |
| GO:0055074 | calcium ion homeostasis | 243 | Adcyap1, Agtr1a, Ccl3, Ccr1, Ccr2, Cxcl2, Cyp27b1, Gnas, Npy2r, Tac1, Tgfb2, | 4.21e-07 |
| GO:0002690 | positive regulation of leukocyte chemotaxis | 43 | Ccl3, Cc17, Ccr1, Ccr2, Sell | 2.64e-06 |
| GO:0002548 | monocyte chemotaxis | 20 | Ccl3, Cc12, Ccr1, Ccr2 | 3.00e-06 |
| GO:0007270 | neuron-neuron synaptic transmission | 102 | Adcyap1, Glra1, Htr1b, Npy2r, Ptgs2, Tac1 | 1.39e-05 |
| GO:0008015 | blood circulation | 297 | Ace, Adcyap1, Agr1a, Alb, Gnas, Hcn4, Ptgs2, Tac1, Tgfb2 | 2.03e-05 |
| GO:2000403 | positive regulation of lymphocyte migration | 11 | Ccl3, Ccl7, Ccr2 | 2.14e-05 |
| GO:0050921 | positive regulation of chemotaxis | 66 | Ccl3, Ccl7, Ccr2, Sell | 2.24e-05 |
| 7 d | ||||
| GO:0002690 | positive regulation of leukocyte chemotaxis | 43 | Ccl2, Ccl3, Ccl4, Ccl7, Ccr2, Cxcl10, Lbp, Sell, Serpine1 | 6.90e-12 |
| GO:0050920 | regulation of chemotaxis | 86 | Ccl2, Ccl3, Ccl4, Ccl7, Ccr2, Cxcl9, Cxcl10,Lbp, Sell, Sema3a, Serpine1 Thbs1 | 8.08e-12 |
| GO:0050921 | positive regulation of chemotaxis | 66 | Ccl2, Ccl3, Ccl4, Ccl7, Ccr2, Cxcl9, Cxcl10,Lbp, Sell, Serpine1 Thbs1 | 1.34e-11 |
| GO:0006955 | immune response | 533 | Ccl2, Ccl3, Ccl4, Ccl7, Ccl12, Ccr2, Cd14, Cd36, Col3a1, Crip1, Cxcl9, Cxcl10, Cxcl11, Fcnb, Il18, Il1rn, Lbp, Lcn2, Serpina3n, Thbs1 | 1.37e-10 |
| GO:0006954 | inflammatory response | 341 | Anxa1, Calca, Ccl1, Ccl3, Ccl4, Ccl7, Ccl12, Ccr2, Cd14, Cxcl10, Il18, Il1rn, Lbp, Serpina3n, Serpine1, Thbs1 | 4.80e-09 |
| GO:0071706 | tumor necrosis factor superfamily cytokine production | 63 | Ccl3, Ccl4, Ccr2, Cd14, Cd36, Il18, Lbp, Thbs1 | 1.70e-07 |
| GO:0016525 | negative regulation of angiogenesis | 46 | Ccl2, Ccr2, Cd36, Cxcl10, Serpine1, Sulf1, Thbs1 | 5.16e-07 |
| GO:0001944 | vasculature development | 422 | Calc1, Ccl2, Ccl12, Ccr2, Cd36, Col3a1, Cxcl10, Il18, Loxl2, Serpine1, Sox18, Srpx2, Sulf1, Tgfbi, Thbs1 | 5.48e-07 |
Ace (angiotensin I converting enzyme (peptidyl-dipeptidase A) 1), Adcyap1 (adenylate cyclase activating polypeptide 1), Agtr1a (angiotensin II receptor, type 1a), Alb (albumin), Anxa1(annexin A1), Calca (Calcitonin-related polypeptide alpha), Ccl2 (chemokine (C-C motif) ligand 2), Ccl3 (chemokine (C-C motif) ligand 3), Ccl4 (chemokine (C-C motif) ligand 4). Ccl7 (chemokine (C-C motif) ligand 7), Ccl12 (chemokine (C-C motif) ligand 12), Ccl24 (chemokine (C-C motif) ligand 24), Ccl7, Ccr41, Ccr2 (chemokine (C-C motif) receptor 2), Cd14 (CD 14 molecule), Cd36 (CD36 molecule (thrombospondin receptor), Col3a1 (collagen, type III, alpha 1), Crip1 (cysteine-rich protein 1), Cxcl2 (chemokine (C-X-C motif) ligand 2), Cxcl9 (chemokine (C-X-C motif) ligand 9), Cxcl10 (chemokine (C-X-C motif) ligand 10), Cxcl11 (chemokine (C-X-C motif) ligand 11), Cyp27b1 (cytochrome P450, family 27, subfamily b, polypeptide 1), Fabp4 (fatty acid binding protein 4, adipocyte), Fcnb (ficolin B), Fgf1 (fibroblast growth factor 1), Glra1 (glycine receptor, alpha 1), Gnas (GNAS complex locus), Htr1b (5-hydroxytryptamine (serotonin) receptor 1B, G protein-coupled), Il18 (nterleukin 18), Il1rn (interleukin 1 receptor antagonist), Lbp (lipopolysaccharide binding protein), Lcn2 (lipocalin 2), Loxl2 (lysyl oxidase-like 2), Npy2r (neuropeptide Y receptor Y2), Olr1 (oxidized low density lipoprotein (lectin-like) receptor 1), Ptgs2 (prostaglandin-endoperoxide synthase 2), Reg3b (regenerating islet-derived 3 beta), S100a9 (S100 calcium binding protein A9), Sell (Selectin L), Sema3a (sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3A), Serpine1 (serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1), Sox18 (SRY (sex determining region Y)-box 18), Srpx2 (sushi-repeat-containing protein, X-linked 2), Sulf1 (sulfatase 1), Tac1 (tachykinin, precursor 1), Tgfb2 (transforming growth factor, beta 2), Thbs1 (thrombospondin 1).
GO, gene ontology.
Table 5.
Effect of Simvastatin and Traumatic Brain Injury (Vehicle/Sham) on Genes of Interest (1.5 Fold Change, p<0.05)
| Simvastatin | Vehicle | |||
|---|---|---|---|---|
| Affymetrix ID | Gene symbol | Genes | Vehicle | Sham |
| 72 h | ||||
| 10739035 | Ace | angiotensin I converting enzyme (peptidyl-dipeptidase A) | 0.62 | 1.85 |
| 10930539 | Adcyap1 | adenylate cyclase activating polypeptide 1 | 0.60 | n.s. |
| 10798194 | Agtr1a | angiotensin II receptor, type 1 | 0.45 | 4.17 |
| 10775968 | Alb | Albumin | 1.85 | 0.49 |
| 10744425 | Alox15 | arachidonate 15-lipoxygenase | 2.83 | n.s. |
| 10736712 | Ccl12 | chemokine (C-C motif) ligand 12 | 1.55 | 1.60 |
| 10745677 | Ccl3 | chemokine (C-C motif) ligand 3 | 1.67 | 2.97 |
| 10736702 | Ccl7 | chemokine (C-C motif) ligand 7 | 1.76 | 10.83 |
| 10757632 | Ccl24 | chemokine (C-C motif) ligand 24 | 2.07 | 1.77 |
| 10921163 | Ccr1 | chemokine (C-C motif) receptor 1 | 1.90 | 4.29 |
| 10914614 | Ccr2 | chemokine (C-C motif) receptor 2 | 1.66 | 13.15 |
| 10775896 | Cxcl2 | chemokine (C-X-C motif) ligand 2 | 2.06 | n.s. |
| 10887947 | Cyp1b1 | cytochrome P450, family 1, subfamily b, polypeptide 1 | 0.62 | 6.77 |
| 10781404 | Dok2 | docking protein 2 | 1.51 | 1.89 |
| 10794734 | F13a1 | coagulation factor XIII, A1 polypeptide | 1.86 | 1.99 |
| 10811126 | Fa2h | fatty acid 2-hydroxylase | 0.66 | n.s. |
| 10814286 | Fabp4 | fatty acid binding protein 4, adipocyte | 1.67 | 4.40 |
| 10804127 | Fgf1 | fibroblast growth factor 1 | 0.63 | 1.73 |
| 10935811 | Gabre | gamma-aminobutyric acid (GABA) A receptor, epsilon | 0.64 | 2.22 |
| 10742820 | Glra1 | glycine receptor, alpha 1 | 0.49 | 5.47 |
| 10842657 | Gnas | GNAS complex locus | 0.65 | 1.83 |
| 10823412 | Gpr149 | G protein-coupled receptor 149 | 0.64 | n.s. |
| 10724315 | Hbb | hemoglobin, beta | 1.65 | n.s. |
| 10724319 | Hbb-b1 | hemoglobin, beta adult major chain | 1.77 | n.s. |
| 10910473 | Hcn4 | hyperpolarization activated cyclic nucleotide-gated potassium channel 4 | 0.52 | 2.01 |
| 10918979 | Htr1b | 5-hydroxytryptamine (serotonin) receptor 1B, G protein-coupled | 0.62 | n.s. |
| 10729791 | Ifit1 | interferon-induced protein with tetratricopeptide repeats 1 | 1.77 | n.s. |
| 10888131 | Kcng3 | potassium voltage-gated channel, subfamily G, member 3 | 0.56 | n.s. |
| 10760290 | Nptx2 | neuronal pentraxin II | 0.59 | n.s. |
| 10815655 | Npy2r | neuropeptide Y receptor Y2 | 0.57 | 3.42 |
| 10866030 | Olr1 | oxidized low density lipoprotein (lectin-like) receptor | 1.55 | 11.06 |
| 10764551 | Ptgs2 | prostaglandin-endoperoxide synthase 2 | 0.63 | 2.60 |
| 10856474 | Reg3b | regenerating islet-derived 3 beta | 0.66 | 2.07 |
| 10824695 | S100a9 | S100 calcium binding protein A9 | 1.74 | 2.23 |
| 10765186 | Sell | selectin L | 1.66 | 4.04 |
| 10874981 | Sulf1 | sulfatase 1 | 0.66 | 1.84 |
| 10853683 | Tac1 | tachykinin, precursor 1 | 0.53 | n.s. |
| 10770577 | Tgfb2 | transforming growth factor, beta 2 | 0.60 | 1.98 |
| 7 days | ||||
| 10729269 | Anxa1 | annexin A1 | 1.64 | 5.84 |
| 10843229 | Cacna1b | calcium channel, voltage-dependent, N type, alpha 1B subunit | 0.64 | 0.66 |
| 10746327 | Cacna1g | calcium channel, voltage-dependent, T type, alpha 1G subunit | 0.65 | n.s. |
| 10725051 | Calca | calcitonin-related polypeptide alpha | 1.72 | n.s. |
| 10907834 | Casp12 | caspase 12 | 1.58 | 2.31 |
| 10736697 | Ccl2 | chemokine (C-C motif) ligand 2 | 2.09 | 13.29 |
| 10745677 | Ccl3 | chemokine (C-C motif) ligand 3 | 1.55 | 2.77 |
| 10736863 | Ccl4 | chemokine (C-C motif) ligand 4 | 1.54 | 1.54 |
| 10736702 | Ccl7 | chemokine (C-C motif) ligand 7 | 2.47 | 1.59 |
| 10736712 | Ccl12 | chemokine (C-C motif) ligand 12 | 1.83 | 1.69 |
| 10914614 | Ccr2 | chemokine (C-C motif) receptor 2 | 1.88 | 4.33 |
| 10803991 | Cd14 | CD14 molecule | 1.90 | 4.41 |
| 10853240 | Cd36 | CD36 molecule (thrombospondin receptor) | 1.74 | 2.83 |
| 10923052 | Col3a1 | collagen, type III, alpha 1 | 1.59 | 2.90 |
| 10887622 | Crip1 | cysteine-rich protein 1 (intestinal) | 1.84 | 1.58 |
| 10771660 | Cxcl9 | chemokine (C-X-C motif) ligand 9 | 1.52 | 1.60 |
| 10771655 | Cxcl10 | chemokine (C-X-C motif) ligand 10 | 1.96 | 6.47 |
| 10771649 | Cxcl11 | chemokine (C-X-C motif) ligand 11 | 1.87 | 4.43 |
| 10845681 | Fap | fibroblast activation protein, alpha | 1.62 | 1.56 |
| 10844005 | Fcnb | ficolin B | 1.58 | 2.72 |
| 10909874 | Il18 | interleukin 18 | 1.59 | 3.21 |
| 10834109 | Il1rn | interleukin 1 receptor antagonist | 1.68 | 2.39 |
| 10734952 | Kcnab3 | potassium voltage-gated channel, shaker-related subfamily, beta member 3 | 0.66 | n.s. |
| 10890609 | Kcnh5 | potassium voltage-gated channel, subfamily H (eag-related), member 5 | 0.54 | n.s. |
| 10904329 | Kcnk9 | potassium channel, subfamily K, member 9 | 0.66 | 0.66 |
| 10903585 | Kcnv1 | potassium channel, subfamily V, member 1 | 0.66 | 0.50 |
| 10841693 | Lbp | lipopolysaccharide binding protein | 1.93 | 2.84 |
| 10844331 | Lcn2 | lipocalin 2 | 1.83 | 5.81 |
| 10781304 | Loxl2 | lysyl oxidase-like 2 | 1.64 | 3.16 |
| 10912439 | Rbp1 | retinol binding protein 1, cellular | 1.52 | 2.92 |
| 10817053 | S100a3 | S100 calcium binding protein A3 | 1.99 | 2.76 |
| 10817057 | S100a4 | S100 calcium-binding protein A4 | 1.69 | 4.37 |
| 10817065 | S100a6 | S100 calcium binding protein A6 | 1.54 | 2.27 |
| 10765186 | Sell | selectin L | 1.73 | 2.02 |
| 10860481 | Sema3a | sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3A | 0.64 | 0.66 |
| 10886621 | Serpina3n | serine (or cysteine) peptidase inhibitor, clade A, member 3N | 2.16 | 4.01 |
| 10761047 | Serpine1 | serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 | 3.05 | 2.29 |
| 10852595 | Sox18 | SRY (sex determining region Y)-box 18 | 1.50 | n.s. |
| 10934865 | Srpx2 | sushi-repeat-containing protein, X-linked 2 | 1.55 | n.s. |
| 10874981 | Sulf1 | sulfatase 1 | 1.53 | n.s. |
| 10797138 | Tgfbi | transforming growth factor, beta induced | 1.63 | 2.25 |
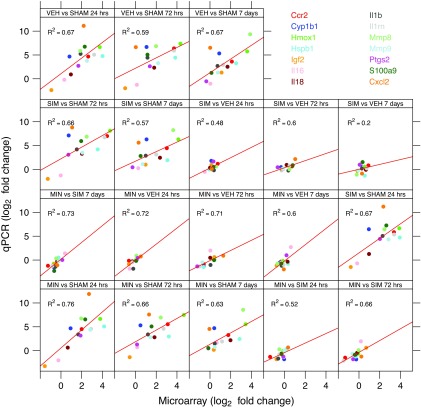
To validate gene expression changes, Figure 5 shows the gene expression of 14 genes selected from specific pathways of interest that are affected by TBI. The data generated via microarray and quantitative-PCR (qPCR) were normalized to GADPH and β-actin. The qPCR findings were highly correlated with the microarray data, the Pearson correlation, were 0.82 and 0.81 when normalized for GADPH and β-actin, respectively.
FIG. 5.
TaqMan based RT-PCR validation of the microarray data for the selected genes: Ccr2 (chemokine (C-C) receptor 2), CxCl2 (chemokine [C-X-C motif], ligand 2), Cyp1b1 (cytochrome P450 1b1), Hmox1 (hemeoxygenase 1), Hspb1 (heat shock protein b1), IGF2 (insulin like growth factor 2), Il1b (interleukin 1 beta), Il16 (interleukin 16), Il18 (interleukin 18), IL1rn (interleukin 1 receptor antagonist), Mmp8 (matrix metallopeptidase 8), Mmp9 (matrix metallopeptidase 9), Ptgs2 (prostaglandin-endoperoxide synthase 2), S100a9 (S100 calcium binding protein A9. The RT-PCR data shown in the figure were normalized to the housekeeping gene -actin.
Discussion
The overall goal of this study was to evaluate the change in transcriptome profiles of brain injured rats as a result of two therapeutic agents and to investigate the correlated behavioral outcomes. The initial dose of minocycline and simvastin resulted in prolonged absorption in the rodents. Therefore, doses were administered 2 h post-injury to attain targeted concentrations in the range by 4 h post-injury. Oral gavage was used to prevent the complication of an additional treatment-induced inflammatory response from the sclerosing properties of minocycline when administered i.p. or subcutaneously.24 Clinically, minocycline is available as a parenteral formulation and can be administered by the intravenous route in patients with TBI resulting in immediate targeted concentrations. Simvastatin is not available in a parenteral formulation and would need to be administered by nasogastric tube clinically, similar to the oral gavage used in this study.
Assuming that the primary effect of minocycline and simvastatin is on attenuating the TBI-induced inflammatory effect, a 72 h treatment duration was chosen. Experimental studies have demonstrated both a neurotoxic as well as neuroprotective function of the inflammatory response.6 The response is pro-inflammatory during the acute phase and anti-inflammatory during the chronic phase, which theoretically assists in the repair and recovery processes.37 The time-dependent dual neurotoxic and neuroprotective effects of the inflammatory response after TBI suggest that the time of initiation and the duration of anti-inflammatory treatment will be essential factors in optimizing therapy. Therefore, a short duration was chosen to target the acute inflammation without reducing the beneficial effects on recovery.6
Transcriptome profiling can be used to evaluate the potential effect of treatment, in addition to increasing the understanding of the mechanism of the treatment effect. Injured rats were compared with sham rats who had undergone anesthesia but no craniotomy to provide an adequate control for the effects of isoflurane anesthesia on gene expression, because it has previously been shown to increase some pro-inflammatory cytokines in the blood.38 Only a time point of 72 h after surgery, however, was used for the sham rats, which may slightly limit the interpretation at the 24 h and 7 day intervals. Minocycline significantly decreased lesion size, although the effect was significantly less than we found with nicotinamide and progesterone in the same CCI model.28 The behavioral outcomes from minocycline and simvastatin showed even less promise. Both drugs improved performance on the locomotor placing task, a measure of fine motor control. Neither drug improved performance on the rotarod (gross locomotor) or the Morris water maze (cognition). This suggests that while there is some benefit to these drugs, there may also be considerable limits in terms of behavioral improvement.
Inflammatory pathways were affected by minocycline only at the 72 h time point. Minocycline decreased the expression of several inflammatory genes, including chemokines and several Mmps. Mmps are increased in TBI and may play a key role in TBIs by degrading components of the basal lamina leading to disruption of the blood–brain barrier.39,40 Specifically, in our CCI model, minocycline decreased the expression of Mmp9 at 72 h post-CCI. In an exploratory clinical trial of minocycline in stroke, minocycline treatment also resulted in decreased serum Mmp9 when measured 72 h after the stroke.41
The majority of the previous work evaluating minocycline in TBI used a close head injury mouse model with 45 mg/kg or 90 mg/kg doses administered i.p. 30 min after injury. Minocycline showed improvement in reducing locomotor hyperactivity, modest gains in rotarod performance, and transient improvement on beam-walking in mice after TBI.9–11,13–16 In the mouse closed head injury model, minocycline 45 mg/kg injected i.p. 30 min post-injury and every 12 h thereafter resulted in differentially expressed Gene Ontology groups involving chemokines, signal transduction, neuronal growth factors, and pro-inflammatory cytokines when evaluated 2, 6, and 24 h post-injury.42 The authors noted a concern about the suitability of minocycline for neuroprotection because minocycline also up-regulated several genes involved in the cell death pathway.
In a CCI rat model, minocycline 50 mg/kg i.p. administered 1 h after injury and then daily for 2 days resulted in a modest improvement of active avoidance and had no effect on myelin loss or preventing cognitive defects.12 Therefore, despite considerable enthusiasm for minocycline as a neuroprotectant in TBI, the majority of the positive pre-clinical studies have used doses initiated immediately after injury. Initiating therapy within 30 minutes of a TBI is not clinically feasible; therefore, minocycline would not provide a practical treatment. A recent study in a mild-blast induced TBI rat model did find that minocycline 50 mg/kg i.p. administered 4 h after injury and then daily for 4 days prevented the development of neurobehavioral abnormalities and normalized serum levels of inflammatory biomarkers.43
One factor that is important to note in comparing this current study to previous studies is that the CCI model is more severe than the weight-drop model used in many of the mouse studies and the mild-blast TBI rat model. In addition, surgery in the present study was also performed with 100% O2 delivered by nose cone, which is common in the field, and may have increased oxidative damage.
Simvastatin has been used in a range of doses from 0.5 mg/kg to 100 mg/kg, with beneficial effects found across the spectrum. Chen and colleagues44 evaluated simvastatin doses ranging from 25 mg/kg to 100 mg/kg administered orally at 1 and 6 h post-TBI and determined that the 37.5 mg/kg dose was the most effective at reducing post-traumatic neurological and motor coordination dysfunction and edema. The majority of the experimental TBI studies have evaluated doses of 0.5 to 1.0 mg/kg/day initiated from 1 to 24 h post-injury. Mahmood and associates45 demonstrated that both the 0.5 and 1.0 mg/kg/day doses administered orally for 14 days starting 1 day post-TBI improved functional outcome. Only the 1 mg/kg dose increased the density of neurons in the hippocampus. In a series of studies evaluating the 1 mg/kg daily for 14 days, the same investigators found beneficial effects on reduction of IL- expression,46 angiogensis,47,48 improved spatial learning,47 reduction of neuronal loss,47 and stimulated neurite outgrowth.49
Our pharmacokinetic studies found that 10 mg/kg every 12 h provided HMG-reductase inhibition activity in the therapeutic range based on pravastatin equivalence. Using HMG-reductase inhibition activity as a measure of simvastatin activity assumes that the neuroprotective effects of simvastatin are correlated with the effects on HMG-reductase, which is a limitation. Our data suggest that the doses needed for HMG-reductase inhibition may be significantly higher than those optimized for neuroprotection. We only evaluated one dosage regiment of simvastatin. Administering a lower dose, initiated at a later time, and/or increasing our duration of treatment may have improved our functional and histological outcomes.
There is evidence for a U-shaped dose-effect relationship (i.e., decreasing effect at higher doses) in TBI for other neuroprotective agents, including rosuvastatin,50 progesterone,51–53 and vitamin D.54 In a TBI murine model, rosuvastatin 1 mg/kg daily for 5 days was effective at reducing neuronal degeneration at 24 h, reducing microgliosis at 7 days post-TBI, and preserving neuronal density at 35 days post-injury.50 A higher dose (5 mg/kg) was ineffective. Therefore, the lack of a significant neuroprotective effect may be because of the high dose of simvastatin or the short duration of treatment.
Both nicotinamide and progesterone demonstrated significant effects on functional recovery of cognition and motor behavior when administered for 72 h in the same CCI model.33,51 Nicotinamide treatment counteracted the changes in genes differentially expressed from TBI,33 while progesterone treatment primarily altered expression of genes not affected directly by TBI itself.51 In contrast, simvastatin significantly increased the expression of inflammatory genes that TBI also increased when administered for 72 h. The gene expression data suggest that simvastatin may have increased the inflammatory response by increasing chemokines, S100a9, and arachidonate 15-lipoxygenase (Alox15) at 24 h. Specifically, Alox15 is considered to have a pro-inflammatory effect and may play a key role in the acute inflammatory response as it reportedly leads to the generation of unstable lipid products from arachidonate.55 The S100 binding proteins are involved in a variety of intracellular calcium dependent functions including cell motility and apoptosis. S100a9 regulates vascular inflammation and contributes to vascular injury by increasing leukocyte recruitment..58 In patients with moderate to severe TBI, increased S-100 concentrations are associated with unfavorable outcomes.58
Conclusion
A major limitation of concentration target dosing based on non-TBI approved indications is the assumption that the neuroprotection effect of the drug occurs at the same concentrations attained in the non-TBI indication. The targeted concentrations will provide information regarding the maximum concentration or dose for drugs with narrow therapeutic ranges but may not be an effective study design for drugs with U-shape pharmacology. In this study, minocycline and simvastatin initiated at dosage regimens designed to produce concentrations in the range reported in patients receiving FDA approved doses did result in significant effects on gene expression in the brain reflecting adequate penetration; however, the dose regimens were only sufficient to produce mild functional neurorestorative effects.
Acknowledgments
The research was supported by a grant from the National Institutes of Health/National Institute of Child, Health and Development (R01 HD061944-01) and by the NIEHS Center for Ecogenetics & Environmental Health (P30ES007033).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Center for Disease Control (2010) Injury prevention and control: Traumatic brain injury Available at: www.cdc.gov/TraumaticBrainInjury/index.html Accessed October11, 2012
- 2.Wang K.K., Larner S.F., Robinson G., and Hayes R.L. (2006). Neuroprotection targets after traumatic brain injury. Curr. Opin. Neurol. 19, 514–519 [DOI] [PubMed] [Google Scholar]
- 3.Jennings J.S., Gerber A.M., and Vallano M.L. (2008). Pharmacological strategies for neuroprotection in traumatic brain injury. Mini Rev. Med. Chem. 8, 689–701 [DOI] [PubMed] [Google Scholar]
- 4.McConeghy K.W., Hatton J., Hughes L., and Cook A.M. (2012). A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 26, 613–636 [DOI] [PubMed] [Google Scholar]
- 5.Holmin S., and Mathiesen T. (1999). Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport 10, 1889–1891 [DOI] [PubMed] [Google Scholar]
- 6.Lenzlinger P.M., Morganti-Kossmann M.C., Laurer H.L., and McIntosh T.K. (2001). The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 24, 169–181 [DOI] [PubMed] [Google Scholar]
- 7.Schouten J.W. (2007). Neuroprotection in traumatic brain injury: A complex struggle against the biology of nature. Curr. Opin. Crit. Care 13, 134–142 [DOI] [PubMed] [Google Scholar]
- 8.Plane J.M., Shen Y., Pleasure D.E., and Deng W. (2010). Prospects for minocycline neuroprotection. Arch. Neurol. 67, 1442–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez Mejia R.O., Ona V.O., Li M., and Friedlander R.M. (2001). Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 48, 1393–1401 [DOI] [PubMed] [Google Scholar]
- 10.Bye N., Habgood M.D., Callaway J.K., Malakooti N., Potter A., Kossmann T., and Morganti-Kossmann M.C. (2007). Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 204, 220–233 [DOI] [PubMed] [Google Scholar]
- 11.Homsi S., Federico F., Croci N., Palmier B., Plotkine M., Marchand-Leroux C., and Jafarian-Tehrani M. (2009). Minocycline effects on cerebral edema: Relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 1291, 122–132 [DOI] [PubMed] [Google Scholar]
- 12.Abdel Baki S.G., Schwab B., Haber M., Fenton A.A., and Bergold P.J. (2010). Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PLoS ONE 5, e12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homsi S., Piaggio T., Croci N., Noble F., Plotkine M., Marchand-Leroux C., and Jafarian-Tehrani M. (2010). Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: A twelve-week follow-up study. J. Neurotrauma 27, 911–921 [DOI] [PubMed] [Google Scholar]
- 14.Siopi E., Calabria S., Plotkine M., Marchand-Leroux C., and Jafarian-Tehrani M. (2012). Minocycline restores olfactory bulb volume and olfactory behavior after traumatic brain injury in mice. J. Neurotrauma 29, 354–361 [DOI] [PubMed] [Google Scholar]
- 15.Siopi E., Cho A.H., Homsi S., Croci N., Plotkine M., Marchand-Leroux C., and Jafarian-Tehrani M. (2011). Minocycline restores sAPPalpha levels and reduces the late histopathological consequences of traumatic brain injury in mice. J. Neurotrauma 28, 2135–2143 [DOI] [PubMed] [Google Scholar]
- 16.Ng S.Y., Semple B.D., Morganti-Kossmann M.C., and Bye N. (2012). Attenuation of microglial activation with minocycline is not associated with changes in neurogenesis after focal traumatic brain injury in adult mice. J. Neurotrauma 29, 1410–1425 [DOI] [PubMed] [Google Scholar]
- 17.Siopi E., Llufriu-Daben G., Fanucchi F., Plotkine M., Marchand-Leroux C., and Jafarian-Tehrani M. (2012). Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: The effect of minocycline. Neurosci. Lett. 511, 110–115 [DOI] [PubMed] [Google Scholar]
- 18.Laufs U., and Adam O. (2012). Acute effects of statins. J. Am. Coll. Cardiol. 59, 71–73 [DOI] [PubMed] [Google Scholar]
- 19.Lu D., Qu C., Goussev A., Jiang H., Lu C., Schallert T., Mahmood A., Chen J., Li Y., and Chopp M. (2007). Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J. Neurotrauma 24, 1132–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H., Lynch J.R., Song P., Yang H.J., Yates R.B., Mace B., Warner D.S., Guyton J.R., and Laskowitz D.T. (2007). Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp. Neurol. 206, 59–69 [DOI] [PubMed] [Google Scholar]
- 21.Li B., Mahmood A., Lu D., Wu H., Xiong Y., Qu C., and Chopp M. (2009). Simvastatin attenuates microglial cells and astrocyte activation and decreases interleukin-1B level after traumatic brain injury. Neurosurgery 65, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiaretti A., Antonelli A., Mastrangelo A., Pezzotti P., Tortorolo L., Tosi F., and Genovese O. (2008). Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J. Neurotrauma 25, 225–234 [DOI] [PubMed] [Google Scholar]
- 23.Wu H., Lu D., Jiang H., Xiong Y., Qu C., Li B., Mahmood A., Zhou D., and Chopp M. (2008). Increase in phosphorylation of Akt and its downstream signaling targets and suppression of apoptosis by simvastatin after traumatic brain injury. J. Neurosurg. 109, 691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Light R.W., Wang N.S., Sassoon C.S., Gruer S.E., and Vargas F.S. (1994). Comparison of the effectiveness of tetracycline and minocycline as pleural sclerosing agents in rabbits. Chest 106, 577–582 [DOI] [PubMed] [Google Scholar]
- 25.Fagan S.C., Edwards D.J., Borlongan C.V., Xu L., Arora A., Feuerstein G., and Hess D.C. (2004). Optimal delivery of minocycline to the brain: Implication for human studies of acute neuroprotection. Exp. Neurol. 186, 248–251 [DOI] [PubMed] [Google Scholar]
- 26.Xu L., Fagan S.C., Waller J.L., Edwards D., Borlongan C.V., Zheng J., Hill W.D., Feuerstein G., and Hess D.C. (2004). Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singhvi S.M., Pan H.Y., Morrison R.A., and Willard D.A. (1990). Disposition of pravastatin sodium, a tissue-selective HMG-CoA reductase inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 29, 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson T.C., Anderson G.D., Kantor E.D., and Hoane M.R. (2012). A comparison of the effects of nicotinamide and progesterone on functional recovery of cognitive behavior following cortical contusion injury in the rat. J. Neurotrauma 29, 2823–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vonder Haar C., Anderson G.D., and Hoane M.R. (2011). Continuous nicotinamide administration improves behavioral recovery and reduces lesion size following bilateral frontal controlled cortical impact injury. Behav. Brain Res. 224, 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martens K.M., Vonder Haar C., Hutsell B.A. and Hoane M.R. (2012). A discrimination task used as a novel method of testing decision-making behavior following traumatic brain injury J. Neurotrauma 29, 2505–2512 [DOI] [PubMed] [Google Scholar]
- 31.Hoane M.R., Pierce J.L., Kaufman N.A., and Beare J.E. (2008). Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxid. Med. Cell. Longev. 1, 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonder Haar C., Emery M.A., and Hoane M.R. (2012). Chronic folic acid administration confers no treatment effects in either a high or low dose following unilateral controlled cortical impact injury in the rat. Restor. Neurol. Neurosci. 30, 291–302 [DOI] [PubMed] [Google Scholar]
- 33.Anderson G.D., Peterson T.C., Farin F.M., Bammler T.K., Beyer R.P., Kantor E.D., and Hoane M.R. (2013). The effect of nicotinamide on gene expression in a traumatic brain injury model. Front. Neurosci. 7, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y., and Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Royal Stat. Soc. Series B 57, 289–300 [Google Scholar]
- 35.Camon E., Magrane M., Barrell D., Lee V., Dimmer E., Maslen J., Binns D., Harte N., Lopez R., and Apweiler R. (2004). The Gene Ontology Annotation (GOA) Database: Sharing knowledge in Uniprot with Gene Ontology. Nucleic Acids Res. 32, D262–D266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentleman R., Carey V., Huber W., Irizarry R., and Dudoit S. (2005). In: Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer Science-Business Media, Inc: New York, pps. 397–420 [Google Scholar]
- 37.Correale J., and Villa A. (2004). The neuroprotective role of inflammation in nervous system injuries. J. Neurol. 251, 1304–1316 [DOI] [PubMed] [Google Scholar]
- 38.Kotani N., Hashimoto H., Sessler D.I., Yasuda T., Ebina T., Muraoka M., and Matsuki A. (1999). Expression of genes for proinflammatory cytokines in alveolar macrophages during propofol and isoflurane anesthesia. Anesth. Analg. 89, 1250–1256 [PubMed] [Google Scholar]
- 39.Hayashi T., Kaneko Y., Yu S., Bae E., Stahl C.E., Kawase T., van Loveren H., Sanberg P.R., and Borlongan C.V. (2009). Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res. 1280, 172–177 [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg G.A. (2002). Matrix metalloproteinases in neuroinflammation. Glia 39, 279–291 [DOI] [PubMed] [Google Scholar]
- 41.Switzer J.A., Hess D.C., Ergul A., Waller J.L., Machado L.S., Portik-Dobos V., Pettigrew L.C., Clark W.M., and Fagan S.C. (2011). Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke 42, 2633–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crack P.J., Gould J., Bye N., Ross S., Ali U., Habgood M.D., Morganti-Kossman C., Saunders N.R., and Hertzog P.J. (2009). The genomic profile of the cerebral cortex after closed head injury in mice: Effects of minocycline. J. Neural Transm. 116, 1–12 [DOI] [PubMed] [Google Scholar]
- 43.Kovesdi E., Kamnaksh A., Wingo D., Ahmed F., Grunberg N.E., Long J.B., Kasper C.E., and Agoston D.V. (2012). Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front. Neurol. 3, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X.R., Besson V.C., Beziaud T., Plotkine M., and Marchand-Leroux C. (2008). Combination therapy with fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, and simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, on experimental traumatic brain injury. J. Pharmacol. Exp. Ther. 326, 966–974 [DOI] [PubMed] [Google Scholar]
- 45.Mahmood A., Goussev A., Kazmi H., Qu C., Lu D., and Chopp M. (2009). Long-term benefits after treatment of traumatic brain injury with simvastatin in rats. Neurosurgery 65, 187–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B., Mahmood A., Lu D., Wu H., Xiong Y., Qu C., and Chopp M. (2009). Simvastatin attenuates microglial cells and astrocyte activation and decreases interleukin-1beta level after traumatic brain injury. Neurosurgery 65, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu D., Qu C., Goussev A., Jiang H., Lu C., Schallert T., Mahmood A., Chen J., Li Y., and Chopp M. (2007). Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J. Neurotrauma 24, 1132–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H., Jiang H., Lu D., Qu C., Xiong Y., Zhou D., Chopp M., and Mahmood A. (2011). Induction of angiogenesis and modulation of vascular endothelial growth factor receptor-2 by simvastatin after traumatic brain injury. Neurosurgery 68, 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H., Mahmood A., Qu C., Xiong Y., and Chopp M. (2012). Simvastatin attenuates axonal injury after experimental traumatic brain injury and promotes neurite outgrowth of primary cortical neurons. Brain Res. 1486, 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Indraswari F., Wang H., Lei B., James M.L., Kernagis D., Warner D.S., Dawson H.N., and Laskowitz D.T. (2012). Statins improve outcome in murine models of intracranial hemorrhage and traumatic brain injury: a translational approach. J. Neurotrauma 29, 1388–1400 [DOI] [PubMed] [Google Scholar]
- 51.Anderson G.D., Farin F.M., Bammler T.K., Beyer R.P., Swan A.A., Wilkerson H.W., Kantor E.D., and Hoane M.R. (2011). The effect of progesterone dose on gene expression after traumatic brain injury. J. Neurotrauma 28, 1827–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cutler S.M., Cekic M., Miller D.M., Wali B., VanLandingham J.W., and Stein D.G. (2007). Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma 24, 1475–1486 [DOI] [PubMed] [Google Scholar]
- 53.Goss C.W., Hoffman S.W., and Stein D.G. (2003). Behavioral effects and anatomic correlates after brain injury: A progesterone dose-response study. Pharmacol. Biochem. Behav. 76, 231–242 [DOI] [PubMed] [Google Scholar]
- 54.Atif F., Sayeed I., Ishrat T., and Stein D.G. (2009). Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol. Med. 15, 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuhn H., and O'Donnell V.B. (2006). Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 45, 334–356 [DOI] [PubMed] [Google Scholar]
- 56.Croce K., Gao H., Wang Y., Mooroka T., Sakuma M., Shi C., Sukhova G.K., Packard R.R., Hogg N., Libby P., and Simon D.I. (2009). Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation 120, 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hermann A., Donato R., Weiger T.M., and Chazin W.J. (2012). S100 calcium binding proteins and ion channels. Front. Pharmacol. 3, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercier E., Boutin A., Lauzier F., Fergusson D.A., Simard J.F., Zarychanski R., Moore L., McIntyre L.A., Archambault P., Lamontagne F., Legare F., Randell E., Nadeau L., Rousseau F., and Turgeon A.F. (2013). Predictive value of S-100beta protein for prognosis in patients with moderate and severe traumatic brain injury: Systematic review and meta-analysis. BMJ 346, f1757. [DOI] [PubMed] [Google Scholar]