Abstract
Invasive candidiasis (IC) is associated with high mortality in intensive care unit (ICU) patients. Timely diagnosis of this potentially fatal condition remains a challenge; on the other hand, the criteria for initiating empirical antifungal therapy in critically ill patients are not well defined in different patient population and ICU settings. Alongside the international guidelines, reaching regional and local consensus on diagnosis and management of IC in ICU setting is essential. This report summarizes our present status of IC management in ICU, considered by a group of Iranian experts in the fields of intensive care and infectious diseases. A round table of 17 experts was held to review the available data and discuss the optimal treatment strategies for IC in critical care setting. Comparative published data on the management of IC were analytically reviewed and the commonly asked questions about the management of IC in ICU were isolated. These questions were interactively discussed by the panel and audience responses were taken to consolidate point-to-point agreement with the panel arriving at consensus in many instances. The responses indicated that patients’ risk stratification, clinical discretion, fungal diagnostic techniques and the empirical therapy for IC are likely to save more patients. Treatment options were recommended to be based on the disease severity, prior azole exposure, and the presence of suspected azole-resistant Candida species. This report was reviewed, edited and discussed by all participants to include further evidence-based insights. The panel expects such endorsed recommendations to be soon formulated for implementation across the country.
Keywords: invasive candidiasis, intensive care unit, therapy, local consensus, Iran
Introduction
Antifungal therapy in high-risk patients with severe sepsis is often started in the absence of proven disease and mainly based on the high clinical suspicion for invasive fungal infections (IFIs).1 The local epidemiology for fungal infections and the prevalence for each of the species at a given care facility may provide physicians with useful clues for the empirical treatment of such infections. In the intensive care unit (ICU) setting, a timely treatment approach for fungal infections is often mandated due to the high mortality attributed to invasive candidiasis (IC) and the lack of precise and reliable diagnostic tools for this condition.2–4 It is often not possible to wait for the culture results and the empirical approach becomes warranted. This treatment strategy is shown to result in reduced IFI-related mortality in ICU.1,3,5
Taken into consideration the international guidelines, arriving at a consensus by Iranian intensive care and infectious diseases experts was deemed necessary in order to improve our current situation in the diagnosis and management of IC in critical care settings. Accordingly, experts from the two disciplines of critical care medicine and infectious diseases attended a round table discussion on 28 July 2013. This report provides a brief review of the literature published by both international and local field authorities on the role which IFI plays in mortality and morbidity of ICU patients. The applicable predictive and diagnostic tools and their place in the clinical management approaches, based on the current international guidelines, were addressed. The consensus from the experts’ panel which is outlined in the present report revolved around three pivotal issues: (1) when to suspect and how to diagnose IFI in ICU setting? (2) when to start prophylactic, preemptive and empirical treatment? and (3) what treatment options to take?
IFIs in ICU: how big is the problem?
IFIs have dramatically increased over the past 20 years. Some contributors to this rise are thought to be the aging population with life sustaining therapies such as widespread use of broad spectrum antimicrobial therapy and the emergence of resistant microorganisms, hemodialysis, widely used invasive medical devices, bone marrow transplantation, solid organ transplantation, chemotherapy regimens and HIV.6
The major risk factors for developing IFI include prior antibiotic use, central venous catheterization, total parenteral nutrition (TPN), a recent major surgery, use of steroids, dialysis and immunosuppression.7 ICU length of stay is considered to be an important risk factor with the rate of infections notably rising after seven to 10 days.8
IC is the fourth leading cause of bloodstream infections and the most common IFI accounting for 70–90% of all invasive mycoses9 with increased cost, morbidity and mortality, especially in ICUs.10 Studies have indicated IC occurring seven to 15 times more frequently than aspergillosis.11 It has been estimated that up to 10% of nosocomial disease is attributed to candida infections12 and almost half of all candida infections occur in ICU.13 In other words, the incidence of IC in ICU appears to be almost 10 times bigger than the medical or surgical wards.10,13,14
Epidemiological insights and the treatment rationale
Candida albicans and Aspergillus fumigatus are the main pathogens responsible for IFIs. In ICU patients however, candida infection accounts for the most prevalent cause of IFIs.1,10 Together with the growing incidence of IC in critically ill patients, the spectrum of pathogenic Candida species has also been changing. Although Candida albicans (accounting for 40–60% of IC) is still the most common pathogen, there has been a significant recent shift towards non-albicans strains, i.e. Candida glabrata, Candida tropicalis and Candida Krusei causing infection in 20–30% of cases.15,16 This change in the epidemiological patterns may at least be partly due to the widespread prophylactic and therapeutic use of fluconazole.17 In ICU patients, infection with C. glabrata is shown to be associated with relatively higher mortality than other Candida spp.15 Studies on the rate of attributable mortality of invasive candidemia in different subtypes have shown that C. glabrata and Candida Krusei are the main subtypes resulting in a mortality rates of more than 50%.18
With an overall estimated mortality rate of 50–75% and 40% for invasive aspergillosis and IC, respectively, IFI imposes a significant healthcare cost burden.19 Particularly in ICU patients, the high incidence rate of IC (9.8 per 1000 admissions) carrying high morbidity (increased length of stay up to 22 days) and mortality rate (almost 30–40%) consumes a significant share of limited resource.20–22 A recent study substantiated that patients with and without candidemia had an ICU crude mortality rate of 52.6% versus 20.6%, respectively (p < 0.001).23
A local report on nosocomial fungal infections in ICU and transplant wards revealed that most prevalent fungal infections appear to result from Candida albicans, Penicillium spp., Aspergillus niger, and Cladosporium spp. where environmental fungal contamination was shown to be more prominent in ICU. Moreover, the length of hospital stay was shown to play a major role in colonization of fungi in critical care settings.24
In other local studies on fungal infections in children with advanced kidney disease undergoing peritoneal dialysis25 and adults with kidney transplantation,26 the role of Candida spp. was shown to be dominant. Furthermore, a multi-centre analysis on the prevalence of IFIs as deep-seated mycosis through direct and culture examinations in immunocompromised hosts (in Tehran, Iran) revealed that Candida spp. tend to be isolated in 70.4% (39.4% C. albicans and 30.9% non-albincans) and Aspergillus spp. in 14.1% of the examined cases.27 Our so far available epidemiological data in Iran appear to be more or less in line with those international rates.28,29
Given the above insights on the prevalence of IFI in critical care setting, the key issue helping to reduce the heavy burden imposed by IC is known to be timely and appropriate interventions. Different studies30,31 have indicated that IFIs’ wide range of mortality rates (22–97%) mainly depends on the time of intervention.
Prompt clinical suspicion and timely diagnosis of IC still remains a challenge as fungal cultures and sensitivity may not be readily available globally, fungal cultures and non-culture-based diagnostic tools lack necessary sensitivities and in many instances awaiting cultures or other paraclinical reports could potentially delay definitive management. Therefore, clinical predictive rules and scoring systems could act as a bridge to prevent detrimental delays in instituting management with its antecedent increase in morbidity and mortality.
With regard to antifungal therapy timing, the empirical approach guided by practical experience, observation and non-specific evidence in a given high-risk patient, is clinically supported by different authors.3,21,22,31 Therefore, the risk factors for IC in critically ill ICU patients should be well defined and the population at highest risk should be targeted promptly for empiric therapy regardless of the availability of the paraclinical reports or definitive diagnosis.32,33 The empirical approach which mostly depends on clinical suspicion is shown to result in better outcome in high-risk patients admitted to ICU.1,3,10
The recent increase in fluconazole-resistant Candida species has encouraged the use of other antifungals such as echinicandins.17,34 The high activity, broad spectrum and low toxicity profile of these agents, of which caspofungin is currently available in Iran, make them ideal agents for empirical use against IC.35,36 The most recent IDSA (Infectious Disease Society of America)35 and ESCMID (European Society for Clinical Microbiology and Infectious Diseases)34 guidelines have recommended the empirical antifungal therapy in critically ill patients with risk factors for IC without any other known cause for fever.
Given the high mortality, other than the empirical approach, prophylactic and preemptive therapy might become indicated in distinct high-risk populations for candida infection.4 With regard to the targeted therapy, upon documentation of candidemia, the infecting strains should be identified for appropriate therapy as Candida species are variably susceptible to different antifungals.37
The above insights reemphasize the clinical burden and the significance of appropriate clinical decision making on therapeutic approaches in IFI within ICU. As substantiated in an earlier consensus report from a group of infectious disease experts in Iran, early initiation of antifungal therapy may reduce the IFI burden in ICU; however, the widespread use of the available therapeutic options should be balanced against their cost and benefits as well as the potential emergence of resistance. After all, the empirical antifungal therapy approach was considered a strongly recommended approach in this report.38
IC in ICU-admitted patients, the implications of available scoring systems
According to a recent survey, 50–80% of the critically ill patients who were admitted to ICU had already been exposed to risk factors for IC, 5–15% had candida colonization on admission and 5–30% actually had IC.29 In the presence of such a clinical prevalence and impact, the diagnosis of IC in critically ill patients remains difficult. Both culture and non-culture-based diagnostic measures are subject to noticeable pitfalls.
Cultures from non-sterile sites are mainly colonization and blood cultures for candidemia become positive in only half of the cases.28 Despite this lack of sensitivity, fungal blood culture remains the gold standard for IC diagnosis. Culture-based diagnosis and species identification is time-consuming and dependence on the culture results only delays therapy.28,39 Nevertheless, newly introduced non-culture-based techniques such as 1,3 -β-d-glucan (1,3-BG) detection, C. albicans germ tube antibodies (CAGTA), Mannan-antigen plus anti-Mannan-antibody measurement, florescent in situ hybridization (FISH), matrix-assisted laser desorption ionization time of flight mass-spectroscopy (MALDITOF-MS) and Pan-fungus polymerase chain reaction (PCR) are shown to provide some clinical value in IFI diagnosis.39 However, many of these techniques have demonstrated unacceptably low sensitivity or specificity and are not commercially available in many settings. These investigatory tools thus need to undergo further trials for their clinical validation.40–42
The 1,3-BG test is shown to have false-positive results among ICU patients with a generally low (<70%) specificity in hospitalized patients. Some of the main attributable factors for the false-positive results of this test are hemodialysis, gauze contamination during surgery, intravenous immunoglobulin (IVIG) administration, bacterial infections, and the use of antimicrobial drugs such as colistin, ertapenem, cefazolin, cefotaxime and ampicillin-sulbactam.39,40
Given the above and based on the earlier insights on the incidence of IC among high-risk ICU patients, some risk prediction models have been developed.5,10,43,44
The most popular prediction rule and scoring system for IC in non-neutropenic adult patients are Ostrosky-Zeichner model45and Candida Score.44 According to Ostrosky-Zeichner prediction rule with the sensitivity, specificity, positive predictive value and negative predictive value of 34%, 90%, 10% and 97%, respectively, patients with indwelling central venous catheters who have received systemic antibiotics and have at least two of the following: TPN, any dialysis, any major surgery, pancreatitis, any steroid therapy or immunosuppression, are considered potentially high risk to develop IC.10,45 Candida Score is based on the presence or absence of conditions (with the coefficient β or individual risk scores in brackets) such as TPN (0.908), surgery on ICU admission (0.997), multi-focal Candida spp. colonization (1.112) and severe sepsis (2.038). Patients with a score of >2.5 are at high risk for development of IC.44 With the sensitivity of 81% and specificity of 74%, one may only need the presence of sepsis and any one of the three other remaining risk factors or the presence of all of them together except sepsis in order to consider starting antifungal treatment for a particular patient.32,44
The clinical suspicion for IC based on these scoring systems can provide physicians with an easy tool for timely administration of an appropriate antifungal agent.44,45
Therapeutic approaches in IC, the position of international guidelines
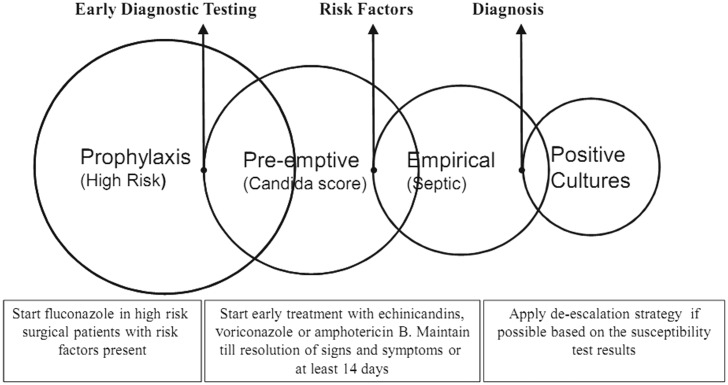
Therapeutic approaches for IC are mainly formulated based on risk factors identification, early diagnostic testing or the definitive diagnosis. As such, patients might receive antifungal agents as prophylaxis, preemptive, empirical or targeted therapy. Figure 1 illustrates a simple scheme for these approaches.31,46,47
Figure 1.
Management strategies for invasive candidiasis. In critically ill and high-risk patients, early initiation of antifungal therapy is shown to reduce mortality. Courtesy of Zaragoza R and Peman J, 2008, subject to creative commons license.
According to the most recent IDSA guideline,35 the empirical therapy against IC in non-neutropenic adult patients should include fluconazole for the less critically ill patient with no recent azole exposure, and echinicandins (caspofungin, micafungin and anidulafungin) for moderate to severely ill patients or those with recent azole exposure. Amphotericin B (AmB) or its lipid formulation (L-AmB) is considered when other antifungals are not tolerated. Given the potential side effects, ESCMID guidelines have not recommended amphotericin B deoxycholate for any indication for the management of IC.34 Table 1 summarizes the therapeutic options for treating IC in non-neutropenic adult patients with moderate to severe illness, based on recommendations from the American Thoracic Society48 and IDSA guidelines.35
Table 1.
Summary of the ATS and IDSA guidelines for treating invasive candidiasis in critically ill patients.
| Disease manifestation | American Thoracic Society (ATS) guideline | Infectious Disease Society of America (IDSA) guideline |
|---|---|---|
| Candidemia, Clinically unstable; moderate to severe illness | Amphotericin B deoxycholate (0.6–1.0 mg/kg/d) or lipid-based amphotericin B (3–5 mg/kg/d) OR Caspofungin (70 mg IV loading dose day 1, then 50 mg/d, IV) OR Micafungin (100 mg/d, IV) OR Anidulafungin (200 mg on day 1, then 100 mg/d, IV) OR Voriconazole (6 mg/kg/12 h x2, then 3 mg/kg/12 h) OR High-dose Fluconazole (800 mg/d) and Amphotericin B (0.6–1.0 mg/kg/d) OR A combination of Fluconazole (800 mg/d) and Amphotericin B (0.6–1.0 mg/kg/d) for the first 5–6 d. | First-line treatment Caspofungin (70 mg IV loading dose day 1, then 50 mg/d, IV) OR Micafungin (100 mg/d, IV) OR Anidulafungin (200 mg on day 1, then 100 mg/d, IV) Alternative regimen Fluconazole 800 mg IV, loading dose; then 400 mg/d, IV or PO |
Recent outcome prediction studies have indicated adverse outcome predictors such as ICU length of stay, renal insufficiency, thrombocytopenia, hematological malignancies, need for mechanical ventilation, needs for inotropic support, APACHE II (Acute Physiology and Chronic Health Evaluation II) score of >20 at the time of candidemia, inadequate empiric antibiotic treatment and delay in starting antifungal therapy.3,31,49
Taken together, since delayed treatment leads to an unacceptably high mortality with a significant human and economic burden,31 prompt diagnosis and management should be sought. Application of the validated scoring systems such as Candida Score44 and Ostrosky-Zeichner model5,10,45 in ICU will help clinicians to identify the high-risk patients who substantially benefit from prompt treatment against IC. Meanwhile, utilizing the evolving diagnostic strategies and modalities as well as the expanding antifungal armamentarium can be justified through continuous research in the field.
Literature review, participants and the consensus method
To gather evidence, we started from a literature review on the management strategies for IFIs in critical care setting. To do so, databases including MEDLINE (PubMed), Scopus and Google Scholar were searched and relevant papers together with the most recent international practice guidelines on the management of IC in ICU were retrieved and circulated amongst invited panelists. All contributors had reviewed the resources before attending this experts’ input forum.
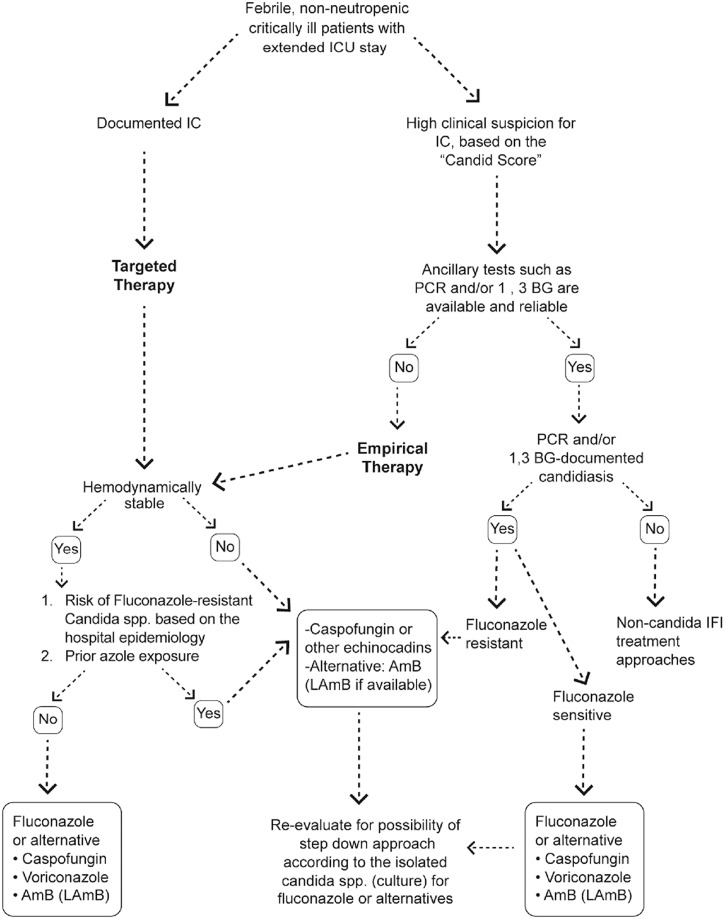
The panel comprised 17 experts (intensive care and infectious diseases specialists) from different medical care centres and universities in Tehran, Iran. Each was invited based on his/her expertise in the management of IC and other fungal infections in critical care setting. Following plenary talks, interactive discussion and review of literature, three pivotal and commonly asked questions were dealt with. Audience responses and supporting evidence were put together to consolidate point-to-point agreement. For most instances, the expert panel could draw an agreed-upon pathway. One of the authors consolidated the experts’ comments and later, circulated the decision checklist amongst the panelists for endorsement. The clinical algorithmic approach which was agreed by the panel to be followed for the management of IC in the critical care setting (as detailed in the result section of the current report) is summarized in Figure 2.
Figure 2.
The algorithmic approach for the management of invasive candidiasis in critical care setting, agreed upon by the Iranian ICU panel of experts. For referencing and further justifications please see the results section. IC: invasive candidiasis; AmB: Amphotericin B; LAmB: Liposomal Amphotericin B; PCR: polymerase chain reaction; 1,3-BG: 1,3-beta-d glucan; IFI: invasive fungal infections.
The manuscript generated from this experts’ meeting was distributed, reviewed and edited by all participants. The current expert opinion report as well as future local recommendation documents on the management of IC in critically ill patients (on the basis of the present consensus) will be formulated as a local recommendation and is expected to be implemented across the country following necessary endorsements from national health authorities and the allied scientific societies.
Results
How to diagnose IC?
Discussion
Since the local epidemiological data on fungal infections in ICU are still lacking, our perspective of the incidence and the impact of IFIs in our ICUs should be further clarified in future investigations. The IC scoring systems and predictive models are not used commonly in our setting and many clinicians seem not to be conversant with these tools. Some believe that due to the relatively low sensitivity of such scoring systems and risk prediction models and the high number needed to treat, ancillary testing such as 1,3-BG and PCR should be utilized instead. On the other hand, many others rely more on the significance of clinical suspicion and the value of Candida Score as one of the most applicable tools for high-risk patient selection.
As such, when ancillary tests are neither available nor reliable, the use of risk prediction models and scoring tools such as ‘Candida Score’ would distinguish patients who are at markedly increased risk for IC. The appropriate treatment can then be empirically administered.
Responses
Majority (13/17) of the panel agreed that due to the lack of availability and reliability (lab-to-lab variation) of such tests, although they are good to have, clinicians should depend on their clinical suspicion and proceed to empirical antifungal therapy against IC. This applies to critically ill patients or those characterized as high risk for IC, based on the available validated scoring systems namely the Candida Score. At the same time, more precise and guideline-oriented clinical evaluations should be implemented to curb the overuse of antifungal agents in the critical care setting.
Introducing these clinical scoring systems to less experienced physicians in the field and increasing their awareness about the use and misuse of antifungals based on the current evidence will be crucial. The ancillary investigations such as PCR and 1,3-BG can also be considered based on their availability, reliability (reference labs) and cost-utility justification (Figure 2).
When to start therapy? The prophylactic, preemptive and empirical approaches
Discussion
The concept of preemptive and empirical therapy could overlap in many situations. When we intend to empirically treat IC in non-neutropenic and non-transplanted patients, some key issues such as how early is early and how long to continue treatment should be made as clear as possible. In an ICU-admitted patient who continues to exhibit signs of systemic inflammatory response following 4–7 days of ICU stay with appropriate and adequate antibiotic therapy, institution of empirical antifungals for IC is warranted. It also holds true for patients just admitted to ICU or transferred from another facility with his/her clinical state over the past 4–7 days prior to ICU transfer consistent with ongoing infection. This discretion however depends on the clinical judgment of the treating physician.4,35
Other than the empirical approach, some non-neutropenic patients are appropriate candidates for prophylactic antifungal therapy. A patient with major abdominal surgery admitted to ICU is a typical example.4
Time to start antifungal therapy for high-risk patients is the crucial point with a significant value in patients’ outcome.31 In a high-risk and critically ill patient, empirical therapy for IC is not only an essential component of the immediate management but also is part of care continuity. When culture results become available and a distinct strain is recovered, the treatment regimen can be adjusted.20
The duration of empirical antifungal therapy should be at least 14 days (14 days since the beginning of therapy or 14 days following a negative culture result).34,35
Responses
All the experts (17/17) agreed that timely treatment against IC in potentially high-risk patients is crucial. When a patient acquires the score of more than 2.5 in Candida Score and stays for over 4–7 days in ICU, empirical therapy should be strongly encouraged. Treatment should continue for at least 14 days (Figure 2).
What treatment options to use? The question of susceptibility, availability and cost versus utility
Discussion
Considering the availability of the treatment options, we should be able to follow the algorithmic approach laid down by the international guidelines. The decisions should however be adjusted and individualized based on specific limitations such as the availability of the treatment options, cost-utility rationale and tolerability of the given regimen in distinct patients. When high-risk patients for IC are stable and the disease is not severe, patients should be treated with fluconazole if not recently exposed to azoles. In case of prior azole exposure or presumed infection with C. glabrata or C. krusei, echinicandins (caspofungin which is the only echinicandin currently available in Iran) will be the antifungal of choice.35
In case the patient who is at risk for IC is critically ill and hemodynamically unstable, caspofungin is the first-line therapy. Although ESCMID guideline34 has recommended not to use AmB for any indication in these population due to its potential side effects, it should still be an alternative modality against IC when other therapies are not available or poorly tolerated. Local studies are needed to evaluate the cost versus utility of the above therapeutic options against IC in ICU.
Responses
A majority of the panelists (15/17) agreed that empirical therapy is indicated in some subset of ICU patients including those with unexplained sepsis. The decision depends on the candida colonization (at multiple sites) and some other risk factors in the absence of any explained cause for fever. Given the correlation between delay in initiation of anti-fungal therapy and mortality in IC patients, everyone agreed that the most effective antifungal agent (as defined by the guidelines) should be employed empirically. Caspofungin, L-AmB and Fluconazole are in turn the mostly recommended antifungals for the empirical therapy against IC in ICU38 (Figure 2).
Conclusive remarks
All panelists participating in the experts’ meeting for the management of IC in critical care setting were in consensus on the need to promptly identify and manage high-risk patients for IC in ICU. Risk stratification, clinical discretion and the use of validated scoring systems such as the Candida Score are the key to choosing the right patients for empirical antifungal therapy against IC.
International guideline clauses seem to be partly applicable to our local practice. Although there were swings away from AmB in such guidelines, it still should remain in the armamentarium of IC management in our ICU practice. Treatment strategies can be modified on a case by case basis depending on recent azole exposure, severity of the underlying illness and the extent of infection.
Declarations
Competing interests
The present report outlines the communications and experts’ opinions during the meeting held on 28 June 2013, Tehran, Iran. The authors declare no competing interest upon data review, talk delivery during the meeting, interactive discussions and preparation of the present report. MTN has been co-affiliated with Behphar Scientific Committee, Behphar Group, Tehran, Iran.
Funding
This meeting received funding and administrative supports from Behestan Darou PJS, Tehran, Iran.
Guarantor
MTN
Ethical approval
Not applicable
Contributorship
Together with MM (moderator and speaker), AN and SMRH as speakers, all authors equally contributed to literature review. MTN drafted the manuscript. MM, AM, SMRH, AAS contributed to critical reversion of the manuscript for important intellectual content. MTN and AAS provided administrative and technical material support. All authors read and approved the final manuscript. Authors are sorted alphabetically since all made almost equal contributions to this publication.
Acknowledgements
Authors would like to thank P Dindoust, SA Hejazi Farahmand, L Nafarieh and M Eisobky for their invaluable contributions.
Provenance
Not commissioned; peer-reviewed by Pota Kalima
References
- 1. Golan Y, Wolf MP, Pauker SG, Wong JB, Hadley S. Empirical anti-Candida therapy among selected patients in the intensive care unit: a cost-effectiveness analysis. Ann Intern Med 2005; 143: 857–869 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong-James D. Invasive Candida species infection: the importance of adequate empirical antifungal therapy. J Antimicrob Chemother 2007; 60: 459–460 [DOI] [PubMed] [Google Scholar]
- 3. Parkins MD, Sabuda DM, Elsayed S, Laupland KB. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother 2007; 60: 613–618 [DOI] [PubMed] [Google Scholar]
- 4. Playford EG, Lipman J, Sorrell TC. Prophylaxis, empirical and preemptive treatment of invasive candidiasis. Curr Opin Crit Care 2010; 16: 470–474 [DOI] [PubMed] [Google Scholar]
- 5. Ostrosky-Zeichner L, Pappas PG, Shoham S, Reboli A, Barron MA, Sims C, et al. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses 2011; 54: 46–51 [DOI] [PubMed] [Google Scholar]
- 6. Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis: Off Publ Infect Dis Soc Am 2003; 37: 634–643 [DOI] [PubMed] [Google Scholar]
- 7. Osorio JJ, Roman AR, Torre-Cisneros J. [Spectrum and risk factors of invasive fungal infection]. Enfermedades Infecciosas y Microbiologia Clinica 2007; 25: 467–476 [DOI] [PubMed] [Google Scholar]
- 8. Dimopoulos G, Karabinis A, Samonis G, Falagas ME. Candidemia in immunocompromised and immunocompetent critically ill patients: a prospective comparative study. Eur J Clin Microbiol Infect Dis: Off Publ Eur Soc Clin Microbiol 2007; 26: 377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamagni TL, Evans BG, Shigematsu M, Johnson EM. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990–9). Epidemiol Infect 2001; 126: 397–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostrosky-Zeichner L, Pappas PG. Invasive candidiasis in the intensive care unit. Crit Care Med 2006; 34: 857–863 [DOI] [PubMed] [Google Scholar]
- 11. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20: 133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheevani, Sharma P and Aggarwal A. Nosocomial Candida infection in a rural tertiary care hospital. J Clin Diagn Res 2013; 7(2): 405–406. [DOI] [PMC free article] [PubMed]
- 13. Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 2005; 366: 1435–1442 [DOI] [PubMed] [Google Scholar]
- 14. Vincent JL, Anaissie E, Bruining H, Demajo W, el-Ebiary M, Haber J, et al. Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med 1998; 24: 206–216 [DOI] [PubMed] [Google Scholar]
- 15. Colombo AL, Nucci M, Salomao R, Branchini ML, Richtmann R, Derossi A, et al. High rate of non-albicans candidemia in Brazilian tertiary care hospitals. Diagn Microbiol Infect Dis 1999; 34: 281–286 [DOI] [PubMed] [Google Scholar]
- 16. Giri S, Kindo AJ. A review of Candida species causing blood stream infection. Indian J Med Microbiol 2012; 30: 270–278 [DOI] [PubMed] [Google Scholar]
- 17. Rocco TR, Reinert SE, Simms HH. Effects of fluconazole administration in critically ill patients: analysis of bacterial and fungal resistance. Arch Surg 2000; 135: 160–165 [DOI] [PubMed] [Google Scholar]
- 18. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis: Off Publ Infect Dis Soc Am 2004; 39: 309–317 [DOI] [PubMed] [Google Scholar]
- 19. Ajenjo HM, Aquevedo SA, Guzman DA, Poggi MH, Calvo AM, Castillo VC, et al. [Epidemiologial profile of invasive candidiasis in intensive care units at a university hospital]. Revista chilena de infectologia: Organo oficial de la Sociedad Chilena de Infectologia 2011; 28: 118–122 [PubMed] [Google Scholar]
- 20. Guo F, Yang Y, Kang Y, Zang B, Cui W, Qin B, et al. Invasive candidiasis in intensive care units in China: a multicentre prospective observational study. J Antimicrob Chemother 2013; 68: 1660–1668 [DOI] [PubMed] [Google Scholar]
- 21. Glockner A, Karthaus M. Current aspects of invasive candidiasis and aspergillosis in adult intensive care patients. Mycoses 2011; 54: 420–433 [DOI] [PubMed] [Google Scholar]
- 22. Kett DH, Azoulay E, Echeverria PM, Vincent JL. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 2011; 39: 665–670 [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez de Molina FJ, Leon C, Ruiz-Santana S, Saavedra P. Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care 2012; 16: R105–R105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kordbacheh P, Zaini F, Kamali F, Ansari K, Safara M. Study on the sources of nosocomial fungal infections at intensive care unit and transplant wards at a teaching hospital in Tehran. Iranian J Publ Health 2005; 34: 1–8 [Google Scholar]
- 25. Hooman N, Madani A, Dorcheh MS, Mahdavi A, Derakhshan A, Gheissari A, et al. Fungal peritonitis in Iranian children on continuous ambulatory peritoneal dialysis; a national experience. Iranian J Kidney Dis 2007; 1: 29–33 [PubMed] [Google Scholar]
- 26. Naeini AE, Sharifi M, Shahidi S, Taheri S, Seirafian S, Taheri D, et al. Intestinal fungal and parasitic infections in kidney transplant recipients: a multi-center study. Saudi J Kidney Dis Transpl 2012; 24: 677–683 [DOI] [PubMed] [Google Scholar]
- 27. Bassiri Jahromi S, Khaksar AA. Deep-seated fungal infections in immunocompromised patients in Iran. Iranian J Allergy Asthma Immunol 2005; 4: 27–32 [PubMed] [Google Scholar]
- 28. Bougnoux ME, Kac G, Aegerter P, d'Enfert C, Fagon JY. Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med 2008; 34: 292–299 [DOI] [PubMed] [Google Scholar]
- 29. Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 2009; 37: 1612–1618 [DOI] [PubMed] [Google Scholar]
- 30. Sinko J, Csomor J, Nikolova R, Lueff S, Krivan G, Remenyi P, et al. Invasive fungal disease in allogeneic hematopoietic stem cell transplant recipients: an autopsy-driven survey. Transpl Infect Dis: Off J Transpl Soc 2008; 10: 106–109 [DOI] [PubMed] [Google Scholar]
- 31. Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005; 49: 3640–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leroy G, Lambiotte F, Thevenin D, Lemaire C, Parmentier E, Devos P, et al. Evaluation of “Candida score” in critically ill patients: a prospective, multicenter, observational, cohort study. Ann Intensive Care 2011; 1: 50–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Troughton JA, Browne G, McAuley DF, Walker MJ, Patterson CC, McMullan R. Prior colonisation with Candida species fails to guide empirical therapy for candidaemia in critically ill adults. J Infect 2010; 61: 403–409 [DOI] [PubMed] [Google Scholar]
- 34. Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis 2012; 18(suppl 7): 19–37 [DOI] [PubMed] [Google Scholar]
- 35. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis: Off Publ Infect Dis Soc Am 2009; 48: 503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. CANCIDAS® (caspofungin acetate). SPC. USPI-I-09910113R002 2013.
- 37. Kanji JN, Laverdiere M, Rotstein C, Walsh TJ, Shah PS, Haider S. Treatment of invasive candidiasis in neutropenic patients: systematic review of randomized controlled treatment trials. Leuk Lymphoma 2013; 54: 1479–1487 [DOI] [PubMed] [Google Scholar]
- 38. Mardani M, Tabarsi P, Yadegarinia D, Talebi Taher M, Najafi N, Hajabdolbaghi M, et al. Treatment of invasive fungal infection: recommendations from scientific leaders' meeting on November 3rd, 2011 Tehran – Iran. Iran J Clin Infect Dis 2011; 6: 179–181 [Google Scholar]
- 39. Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis 2005; 5: 609–622 [DOI] [PubMed] [Google Scholar]
- 40. Hanson KE, Pfeiffer CD, Lease ED, Balch AH, Zaas AK, Perfect JR, et al. Beta-d-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study. PloS One 2012; 7: e42282–e42282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trovato L, Betta P, Romeo MG, Oliveri S. Detection of fungal DNA in lysis-centrifugation blood culture for the diagnosis of invasive candidiasis in neonatal patients. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis 2012; 18: E63–E65 [DOI] [PubMed] [Google Scholar]
- 42. Held J, Kohlberger I, Rappold E, Busse Grawitz A, Hacker G. Comparison of (1->3)-beta-d-glucan, mannan/anti-mannan antibodies, and Cand-Tec Candida antigen as serum biomarkers for candidemia. J Clin Microbiol 2013; 51: 1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hermsen ED, Zapapas MK, Maiefski M, Rupp ME, Freifeld AG, Kalil AC. Validation and comparison of clinical prediction rules for invasive candidiasis in intensive care unit patients: a matched case-control study. Crit Care 2011; 15: R198–R198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leon C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med 2006; 34: 730–737 [DOI] [PubMed] [Google Scholar]
- 45. Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis: Off Publ Eur Soc Clin Microbiol 2007; 26: 271–276 [DOI] [PubMed] [Google Scholar]
- 46. Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis: Off Publ Infect Dis Soc Am 2006; 43: 25–31 [DOI] [PubMed] [Google Scholar]
- 47. Zaragoza R, Peman J. The diagnostic and therapeutic approach to fungal infections in critical care settings. Adv Sepsis 2008; 6: 90–98 [Google Scholar]
- 48. Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, et al. An official American thoracic society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med 2011; 183: 96–128 [DOI] [PubMed] [Google Scholar]
- 49. Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005; 43: 1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]