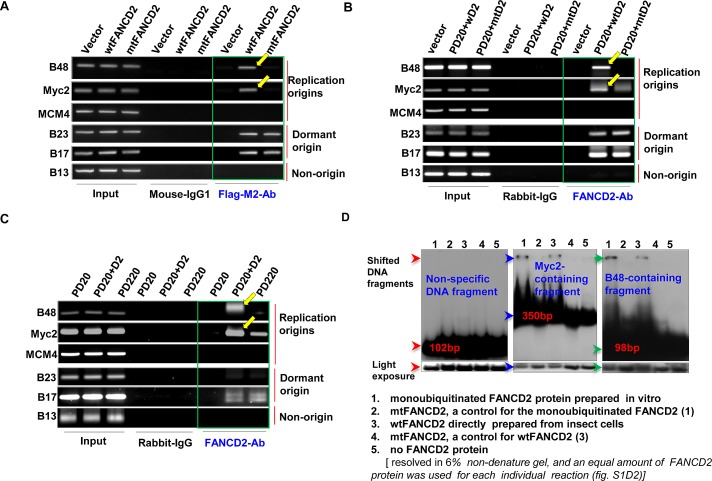
Figure 1. Monoubiquitinated FANCD2 strongly interacts with replication origins in vivo and in vitro.
Flag-wtFANCD2, but not Flag-mtFANCD2, strongly interacts with B48 and Myc2 replication origins in normally growing HEK293 cells. HEK293 cells were transiently transfected with empty vector, Flag-wtFANCD2 and Flag-mtFANCD2. 48 hr post-transfection, cells were fixed with 1% paraformaldehyde for ChIP assay. ChIP PCR primers for five origins were designed upon published sequences. As yellow arrowheads pointed, Flag antibodies pulled down a low amount of origin B48 or Myc2 -containing DNA fragments from the lysates of cells transfected with Flag-mtFANCD2, as compared to the cells transfected with Flag-wtFANCD2. Plasmids used were equally transfected into cells (Supplementary Fig. 1A2-4). Mouse-IgG1 was used as IP control and did not pull down any noticeable amount of DNA fragment. 5% of the IP-lysate was used to prepare PCR template as an input control. B. WtFANCD2, but not K561R mtFANCD2, clearly interacts with the replication origins in FA derivative cells. PD20 FA cells (FANCD2-/-) were used to establish stably-transfected cells carrying wtFANCD2 or mtFANCD2/inactivated FANCD2 with a similar cell proliferation profile with an exception of 2-3% less in S-phase population upon cells carry inactivated FANCD2 (Supplementary Fig. 1C1, c2). ChIP assays on these FA derivative cells [expressing a comparable level of FANCD2 protein (Supplementary Fig. 1C3)] were performed by using FANCD2 antibodies, which can pull down more B48 and Myc2 origin-containing DNA fragments from the lysates of cells carrying wtFANCD2, as compared to cells harboring mtFANCD2 (D2 labeled in the figure means FANCD2). C. FANCD2 is insufficiently associated with B48 and Myc2 origins in PD220 FA cells (FANCA-/-). FANCD2 ChIP assays on PD20, PD20+wtFANCD2, or PD220 cells were conducted. Equal amount of FANCD2 protein levels was confirmed in PD20+wtFACD2 cells and PD220 cells (Supplementary Fig. 1C3). FANCD2 antibodies can pull down more B48 and Myc2 origin-containing DNA fragments in PD20+wtFANCD2 cells not in PD220 cells (as a result of FANCA-/-, FANCD2 cannot be monoubiquitinated). D. Myc2 and B48 origin-containing DNA fragments strongly bind to monoubiquitinated FANCD2, but not un-monoubiquitinated or mtFANCD2. Ubiquitin-conjugated or –unconjugated FANCD2 and mtFANCD2 proteins (Supplementary Fig. 1D) were incubated with 32p-labeled DNA segments containing B48, Myc2, MCM4, B23, B17 or B13. The resulting complexes were analyzed on a 6% polyacrylamide non-denature gel. As pointed by blue and green arrowheads, B48 and Myc2 strongly bind to monoubiquitinated FANCD2 among all forms of FANCD2 proteins tested, indicated by the shifted 32p-labled DNA segments. Like non-origin fragment B13 (shown), B23 and B17, as well as MCM4 appeared to be incapable of binding well to any forms of FANCD2 proteins under the assay condition here used (not shown).