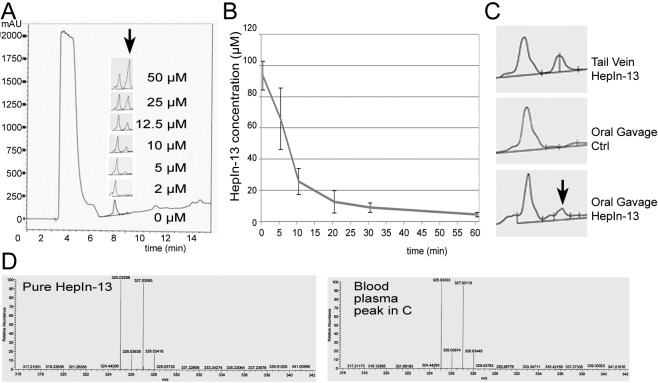
Figure 3. Pharmacokinetics and oral bioavailability of the Hepsin inhibitor HepIn-13.
(A) Analytical HPLC profiles (mAU at 220 nm) of HepIn-13 which was dissolved in the mouse blood plasma at indicated concentrations and then extracted and analyzed by HPLC. (B) Blood concentrations of HepIn-13 at indicated time points after tail vein injection of 1 mg of the compound. Data are the means of three independent experiments ±SD. (C) HepIn-13 is orally bioavailable. Analytical HPLC profiles (mAU at 220 nm) of blood plasma extracts: from tail vein injected with HepIn-13, untreated and oral gavage treated mice. (D) Mass Spectrometry confirmation of the presence of HepIn-13 in blood plasma in the peak highlighted by arrow in panel C.