INTRODUCTION
Supracricoid partial laryngectomy (SCPL) has become the preferred treatment alternative for patients with early to intermediate stage squamous cell carcinoma (SCC) of the larynx. In the United States, the standard of care for patients with early disease is radiation therapy, whereas combined chemoradiation remains the usual treatment recommendation for patients with intermediate and advanced disease. Unfortunately, severe dysphagia often results from radiation-induced fibrosis which may be exacerbated with the addition of chemotherapy.1-2 With rehabilitation, total laryngectomy usually results in acceptable speech and swallowing; however, the stigma associated with a tracheostoma is a significant concern to patients and their families.3-4 Alternatively, SCPL is associated with high local control rates and allows preservation of speech and swallowing without a permanent tracheostomy.
SCPL is an aggressive surgical procedure. Depending on the extent and primary site of the tumor, SCPL involves resection of the entire thyroid cartilage, both true vocal folds, and both false vocal folds. In effect, the glottic sphincter, an essential component of airway protection during normal deglutition, is completely removed.5 Despite this, the procedure is associated with a low incidence of medical complications and high rates of swallowing recovery between 86% and 100% in published reports .5-10 Although these outcomes are extremely favorable, swallowing data are largely based on anecdotal information and small case series, mostly in Europe.6, 9-10 Therefore, the purpose of this study was to objectively describe swallowing physiology in patients treated with SCPL using scientific methodology. We also examined potential associations between surgical variables and three postsurgical outcomes: (1) medical course of recovery, (2) swallowing physiology and therapy effectiveness and (3) nutritional outcomes. We believe that these data will allow us to identify critical treatment variables that are important to the functional success of patients who are treated with SCPL and will help identify the most effective swallowing strategies during the acute period of recovery.
MATERIALS AND METHODS
We retrospectively analyzed the medical records of all patients who underwent SCPL at The University of Texas M.D. Anderson Cancer Center from September, 1997 to March, 2005. This protocol was approved by the Institutional Review Board and a waiver of informed consent was obtained. The cohort comprised two groups of patients. The first group included those with previously untreated T2 – T4 laryngeal carcinoma treated with induction chemotherapy and SCPL. The second group included those who were initially treated with definitive radiation and underwent SCPL for surgical salvage. We collected data on patients’ length of hospital stay, time to tracheotomy decannulation and complication rates to determine medical course of recovery. Time to oral intake, feeding tube dependency and final diet level defined nutritional outcomes. An oral diet was defined as the ability to eat regular or soft foods by mouth without liquid restrictions.
Patients were routinely referred to the Speech Pathology Section for pre-operative evaluation and counseling and were followed postoperatively for swallowing examination and treatment. Data collection included dysphagia symptoms (aspiration and pharyngeal residue) and physiologic deficits (neoglottic competency, hyolaryngeal excursion and base of tongue motion) observed during instrumental evaluation, the modified barium swallow (MBS) study. Base of tongue motion, ability to oppose to the posterior pharyngeal wall, and hyolaryngeal excursion, the superior and anterior movement of the larynx and hyoid complex, were selected as key components to determine adequate pharyngeal transit and airway protection during swallowing.2 Additionally, the use and effectiveness of compensatory strategies on swallowing function were evaluated. All MBS studies were conducted in standard format with thin, thick and solid consistencies, as previously described.11 Every patient attempted thin liquids during MBS studies; however, other consistencies were presented based on the patient’s response and tolerance, at the discretion of the treating clinician. Compensatory strategies and therapeutic intervention were selected and attempted on the basis of the patient’s swallowing response to each food type during the MBS study.
Outcomes were evaluated on the basis of the type of reconstruction following SCPL including cricohyoidoepiglottopexy (CHEP), cricohyoidopexy (CHP), and tracheocricohyoidopexy (TCHP), the presence or absence of arytenoid resection, and the receipt of radiotherapy. For the purpose of analysis, arytenoid resection was defined as resection of part or all of one arytenoid cartilage with or without mucosal preservation. Although some patients received more than 2 MBS studies postoperatively, swallowing outcomes were based on findings from the first and second MBS studies only.
Reconstructive Technique
A review of the surgical technique employed at The University of Texas M.D. Anderson Cancer Center was recently published.12 Briefly, the technique preserves the superior laryngeal nerve as well as the recurrent laryngeal nerve. During reconstruction, the arytenoid cartilages must be resuspended anteriorly to permit reapproximation to the epiglottis and/or tongue base. The fascia of the inferior constrictors should be resuspended as well, which effectively recreates the pyriform sinuses and the lateral gutters during pharyngeal phase of deglutition. Above all, a tight impaction from the cricoid cartilage to the epiglottis (CHEP) and/or tongue base (CHP) must be achieved.
Statistical Analysis
Comparisons between treatment groups in which the endpoint was categorical were assessed with Pearson’s chi-square test or, where there were fewer than 10 subjects in any cell of a 2×2 table, with the two-tailed Fisher’s exact test. Where endpoints were measured in terms of time intervals and there were no null values, the treatments were compared by using the Tukey Honest Significant Difference test for unequal numbers in each group. The length of time for hospital stay and time to tracheotomy decannulation were compared between the three surgical reconstruction groups using the Kruskal-Wallis analysis of variance by ranks test because the numbers in each group were different and the variances between groups were not homogeneous. The time to feeding tube removal was compared between treatment groups using log rank tests. For patients whose feeding tubes were not removed during the period of observation, the intervals from surgery to last contact were used as censored values in the calculations. All statistical analyses were performed using the Statistica for Windows software package (StatSoft, Tulsa, OK).
RESULTS
Patient Population
Twenty-seven patients, 24 males and 3 females, comprised the study population. Seventeen patients had recurrent laryngeal carcinoma and received radiation therapy as definitive treatment prior to salvage resection. Ten patients had SCPL after induction chemotherapy using paclitaxel, ifosfamide, and cisplatin (TIP) as part of a prospective clinical trial.13 Details of the patient population are presented in Table 1.
Table 1.
Patient Population
| Sex | |
| Male | 24 (88.9%) |
| Female | 3 (11.1%) |
| Age (yrs) | |
| Mean (SD) | 59 (8.9) |
| Tumor Site | |
| Glottic | 20 (74.1%) |
| Supraglottic | 7 (25.9%) |
| T-classification | |
| 2 | 5 (18.5%) |
| 3 | 3 (11.1%) |
| 4 | 2 (7.4%) |
| Recurrent | 17 (63.0%) |
| Treatment | |
| Induction+SCPL | 10 (37.0%) |
| XRT+Salvage SCPL | 17 (63.0%) |
| Reconstruction | |
| CHEP | 20 (74.1%) |
| CHP | 5 (18.5%) |
| TCHP | 2 (7.4%) |
| Arytenoid resection | |
| Yes | 8 (29.6%) |
| No | 19 (70.4%) |
Patients were seen postoperatively by the speech pathologist an average of six times (SD: 4; median: 5; range: 1-21). The average length of follow-up was 28 months from the date of SCPL (SD: 24.2; median: 15.0; range: 3-90). All patients had a nasogastric tube placed perioperatively. No patient underwent prophylactic placement of a gastrostomy tube. All swallowing results and nutritional outcomes data were obtained prior to development of locoregional recurrence.
Medical Course of Recovery
The patients’ hospitalization course and rates of complications are presented in Table 2. The type of SCPL had no significant effect on the course of recovery. Although length of hospitalization and tracheostomy tube dependence were slightly greater in patients who were irradiated or required arytenoid resection, neither radiation nor arytenoid resection significantly affected patients’ postoperative course including length of stay and time to decannulation.
Table 2.
Medical course of recovery
| Course of recovery | Complications | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| Length of stay (days) | Time to Decannulation (wks) |
Any | Subcutaneous Emphysema |
Pneumonia | |||||||||||||
|
|
|||||||||||||||||
| N | Mean | SD | p | N | Mean | SD | p | Ratio | % | p | Ratio | % | p | Ratio | % | p | |
| CHEP | 20 | 8.0 | 10.7 | 20 | 5.0 | 8.8 | 14/20 | 70.0 | 6/20 | 30.0 | 6/20 | 30.0 | |||||
| CHP | 5 | 6.4 | 1.8 | 5 | 7.2 | 6.5 | 2/5 | 40.0 | 1/5 | 20.0 | 1/5 | 20.0 | |||||
| TCHP | 2 | 8.5 | 0.7 | 0.167‡ | 1 | - | - | 0.3403‡ | 1/2 | 50.0 | 0.428† | 0/2 | 0.0 | 0.617† | 0/2 | 0.0 | 0.617† |
|
| |||||||||||||||||
| − XRT | 10 | 6.1 | 1.8 | 10 | 3.3 | 5.0 | 6/10 | 60.0 | 3/10 | 30 | 0/10 | 0.0 | |||||
| + XRT | 17 | 8.6 | 11.6 | 0.547* | 16 | 6.6 | 9.7 | 0.377* | 11/17 | 64.7 | 1.000** | 4/17 | 23.5 | 1.000** | 7/17 | 41.2 | 0.022** |
|
| |||||||||||||||||
| − Aryt. resect. | 19 | 5.9 | 1.9 | 18 | 3.8 | 6.9 | 12/19 | 63.2 | 6/19 | 31.6 | 4/19 | 21.1 | |||||
| + Aryt. resect. | 8 | 11.9 | 16.7 | 0.199* | 8 | 8.7 | 10.3 | 0.241* | 5/8 | 62.5 | 1.000** | 1/8 | 12.5 | 0.633** | 3/8 | 37.5 | 0.633** |
|
| |||||||||||||||||
| All SCPL patients | 27 | 7.7 | 9.2 | 26 | 5.3 | 8.2 | 17/27 | 63.0 | 7/27 | 25.9 | 7/27 | 25.9 | |||||
Kruskal-Wallis ANOVA by Ranks
Pearson Chi-square test
Tukey Honest Significant Difference
Two-tailed Fisher Exact test
The most common postoperative complications were pneumonia and subcutaneous emphysema. Other complications included seroma, hematoma, infection, and laryngocele. One patient experienced a cardiac complication and cerebrovascular accident in the acute postoperative period without long-term residual deficits. The type of SCPL or arytenoid resection did not significantly affect the rate of any complication (0.428 and 1.000, respectively), whereas, only patients who were previously irradiated (7/27) developed pneumonia (p = 0.022).
Fourteen of 27 patients required placement of a gastrostomy tube postoperatively mostly due to the severity of aspiration (8/14). Two patients had complications related to their nasogastric tube, and the other four patients had comorbidities that required conversion to a gastrostomy tube.
Swallowing Outcomes
Twenty-two patients were referred postoperatively for videofluoroscopic evaluation of swallowing and underwent at least one MBS study. Eighteen of the 22 patients had 2 MBS studies, seven required three studies, and four patients required four MBS examinations. Initial examinations (MBS1) for 21 patients were conducted, on average, 4 weeks postoperatively (SD: 2.7; median: 3.1; range: 1-9.5). One patient, who was initially lost to follow-up, returned 2-years (110 weeks) postoperatively for MBS examination.
Sixty-seven percent of the patients (18/22) underwent a second MBS study (MBS2) on average 7 weeks after the first examination (SD: 7.6; median: 4.7; range: 2-33). Four of the 22 patients did not undergo an additional swallowing study; three of the four were able to swallow safely without aspiration using a swallowing maneuver and one patient subsequently underwent total laryngectomy because of recurrent disease.
Swallowing Physiology and Symptoms
Neoglottic incompetency resulting in aspiration of liquids during and after the swallow was identified in all patients during MBS1. Fifty-five percent of patients (12/22) aspirated foods (either pureed or soft-solid consistency) in addition to liquids. Ninety-four percent of patients (17/18) continued to aspirate liquids (p=0.4500) while only 33% of patients (6/18) continued to aspirate foods on MBS2 (p=0.2157).
During MBS1, 73% of patients (16/22) were sensate to the aspirate and coughed spontaneously but the remaining patients (27%, 6/22) had no response to the aspirate and in effect, silently aspirated, without coughing or any other response. Six of the 18 patients who underwent a second MBS study silently aspirated during MBS2 Two of those patients also silently aspirated on MBS1, and four patients were identified as new silent aspirators on MBS2 because they were sensate to the aspirate on MBS1.
Reduced hyolaryngeal excursion was identified in 45% of patients (10/22) while decreased base of tongue retraction to the posterior pharyngeal wall was documented in 27% of patients (6/22) on MBS1. These findings were similar on MBS2. Pharyngeal residue was identified in 18% of patients (4/22) on MBS1 and 44% of patients (8/18) on MBS2. Swallowing physiology and symptoms are presented in Figure 1.
Figure 1.
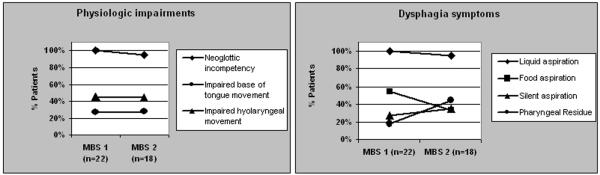
Dysphagia symptoms and physiologic findings during MBS studies
Effects of Swallowing Interventions
The use of swallowing strategies reduced or eliminated aspiration in 64% of patients (14/22) who aspirated during MBS1. Diet modifications alone did not consistently reduce or prevent aspiration. Of all the strategies attempted, the supraglottic swallow maneuver was effective in the most patients (57%, 8/14). When the supraglottic swallow failed to reduce or eliminate aspiration, other swallowing strategies were selected on the basis of the physiologic disorder and the surgical defect. Eight of 22 patients continued to aspirate despite the use of any swallowing strategy. Figure 2 presents all of the strategies recommended for patients who aspirated during MBS1 and MBS2.
Figure 2.
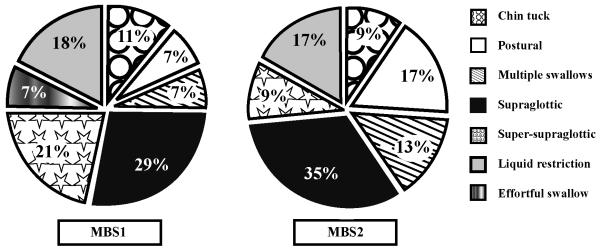
Swallowing strategies recommended during MBS evaluations
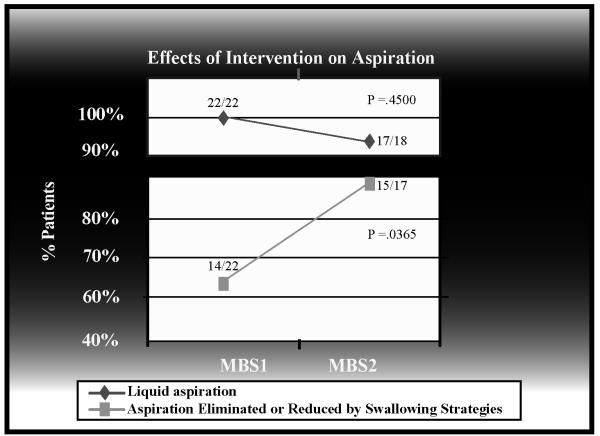
Although the rate of aspiration did not significantly change between MBS1 and MBS2 (100% and 94%, respectively; p=0.4500), there was a statistically significant increase in the ability of swallowing strategies to reduce or eliminate aspiration (p=0.0365) between MBS1 (64%, 14/22) and MBS2 (88%, 15/17). Figure 3 demonstrates rates of aspiration and the effectiveness of swallowing strategies.
Figure 3.
Rates of aspiration and the effectiveness of swallowing strategies
Effect of Type of SCPL, Arytenoid Resection, and Radiation Therapy on Aspiration and Final Nutritional Outcomes
Ultimately, 81% of patients (22/27) maintained oral nutrition with feeding tube removal occurring at a median of 9.4 weeks after surgery. Five patients remained partially (4/27) or fully (1/27) tube dependent. The type of SCPL, the presence or absence of arytenoid resection, or radiation therapy had no statistically significant effect on the occurrence of aspiration or on the patients’ final nutritional outcomes (p=0.3848, p=0.294, and p=0.415, respectively). Table 3 summarizes these results.
Table 3.
Swallowing outcomes by surgery type and radiotherapy
| Aspiration* | Final Nutritional Outcomes | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||
| Yes | Silent | Swallowing Strategies** |
P.O Reg/Soft |
Partial Tube | NPO | Feeding Tube Removal (wks) |
||||||||||||
| Effective | Not effective | |||||||||||||||||
|
|
||||||||||||||||||
| # | # | % | p*** | # | % | # | % | p*** | # | % | # | % | # | % | p**** | Median | p† | |
| CHEP (n=20) | 14 | 5 | 35.7 | 14 | 100.0 | 0 | 0.0 | 17 | 85.0 | 3 | 15.0 | 0 | 0.0 | 5.13 | ||||
| CHP (n=5) | 3 | 1 | 33.3 | 1.000 | 1 | 33.3 | 2 | 66.7 | 0.022 | 3 | 60.0 | 1 | 20.0 | 1 | 20.0 | 29.06 | ||
| TCHP (n=2) | - | - | - | - | - | - | - | - | - | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0.385 | 18.52 | 0.130 |
|
| ||||||||||||||||||
| − XRT (n=10) | 6 | 1 | 16.7 | 5 | 83.3 | 1 | 16.7 | 8 | 80.0 | 2 | 20.0 | 0 | 0.0 | 5.41 | ||||
| + XRT (n=17) | 11 | 5 | 45.5 | 0.333 | 10 | 90.9 | 1 | 9.1 | 1.000 | 14 | 82.4 | 2 | 11.8 | 1 | 5.9 | 0.415 | 6.84 | 0.979 |
|
| ||||||||||||||||||
| − Aryt. resect. (n=i9) | 11 | 4 | 36.4 | 10 | 90.9 | 1 | 9.1 | 16 | 84.2 | 3 | 15.8 | 0 | 0.0 | 5.84 | ||||
| + Aryt. resect. (n=8) | 6 | 2 | 33.3 | 1.000 | 5 | 83.3 | 1 | 16.7 | 1.000 | 6 | 75.0 | 1 | 12.5 | 1 | 12.5 | 0.294 | 12.89 | 0.663 |
| Partial (n=4) | 3 | 2 | 66.7 | 2 | 66.7 | 1 | 33.3 | 2 | 50.0 | 1 | 25.0 | 1 | 25.0 | 22.8 | ||||
| Complete (n=4) | 3 | 0 | 0.0 | 0.400 | 3 | 100.0 | 0 | 0.0 | 0.400 | 4 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0.214 | 4.7 | |
|
| ||||||||||||||||||
| All SCPL patients (N=27) | 17 | 6 | 15 | 2 | 22 | 81.5 | 4 | 14.8 | 1 | 3.7 | 9.38 | |||||||
Aspiration rates based on findings during the second post-operative MBS study (n=18)
Effective swallowing strategies reduced or eliminated the occurrence of aspiration
Two tailed Fisher Exact Test
Pearson chi-square test
Log-rank test
DISCUSSION
The results of this study support the findings of other investigators that SCPL initially results in severe swallowing dysfunction, most notably aspiration, but permits the eventual return to oral nutrition for most patients.5-10 Unlike other studies, however, this investigation retrospectively analyzed course of recovery and swallowing outcomes on the basis of objective instrumental analysis. To our knowledge, this is the first detailed scientific examination that delineates the specific postoperative swallowing physiology and symptoms associated with surgical defects after SCPL. Furthermore, our study is the first to report on the effectiveness of compensatory swallowing strategies in alleviating aspiration to allow patients to safely return to oral nutrition after SCPL. Eighty-one percent of our patients ultimately returned to oral nutrition.
Similar to the findings of other investigations, this study confirms the high incidence of severe aspiration in the immediate postoperative period following SCPL.8,10,14 In our institution, patients generally begin to eat orally despite their aspiration but are closely monitored. However, we rely on the findings from instrumental testing, specifically, the MBS study to help determine patient readiness for oral intake. This highlights two important points. First, appropriate patient selection is crucial to successful hospital recovery and optimal functional outcomes following SCPL. In our study, the most common postoperative complications were pneumonia and subcutaneous emphysema. It is therefore critical to select patients who are able to tolerate the effects of gross aspiration on pulmonary function during the postoperative healing period. Pretreatment speech and swallowing assessment is essential to help determine the patient’s ability to participate in intensive postoperative rehabilitative efforts.
Second, we highly recommend the use of an instrumental swallowing examination to guide the postoperative rehabilitative course of functional recovery. We use the MBS examination because it allows us to objectively assess the amount, severity, and etiology of the aspiration and to determine the patient’s ability to protect the airway using appropriately selected compensatory strategies. Experience has shown that readiness to begin oral intake varies among patients following SCPL. Objective swallowing data provide the clinician with accurate information on which to base recommendations regarding when to begin oral intake and the types and quantities of foods the patient will best tolerate. This is particularly critical in populations that may be at higher risk for complications related to aspiration.
Based on our retrospective analysis, on average, our patients were seen by the speech pathologist 6 times including diagnostic and treatment sessions. At M. D. Anderson Cancer Center, our rehabilitative program follows a three phase model of functional recovery. Phase 1, Pre-oral, occurs in the acute postoperative period prior to referral for MBS1. The goal during Phase 1 is to re-establish neoglottic airflow and competency. Phase I incorporates an exercise protocol that targets laryngeal excursion, neoglottic adduction and airway protection. Patients are decannulated during this phase as tolerated. Phase 2, PO Readiness, generally occurs two to four weeks after surgery with the goal of initiating oral intake while minimizing the occurrence of aspiration. MBS1 is performed and swallowing strategies are selected based on test findings. Phase 3, Functional Return, generally takes place four to six weeks after MBS1. At this point, patients are advanced to the least restrictive diet they are able to tolerate. The goal is to eliminate swallowing strategies as tolerated. We perform MBS2 and tailor the rehabilitation program based on test findings. Patients will receive additional MBS studies on the basis of need.
Our findings showed that the type of SCPL (CHP, CHEP, or TCHP), the presence or absence of arytenoid resection or radiation therapy had no statistically significant effect on the occurrence of aspiration. It is possible, however that the extent of arytenoid resection may have an effect on swallowing recovery and final outcome. The number of patients in our study who had partial arytenoid resection was too few to reliably analyze. We are, therefore, reticent to draw conclusions from a retrospective review based on such a limited sample size. Patients who had any amount of arytenoid resection were grouped together for analysis, and no significant effect on swallowing outcomes was found. However, further investigation is needed to evaluate the relationship between the extent of arytenoid resection during SCPL and swallowing outcomes.
Likewise, the type of SCPL or the ability to preserve both arytenoid cartilages did not significantly affect the occurrence of complications (p= 0.617 and p=0.633, respectively). However pneumonia secondary to aspiration occurred only in patients who were previously irradiated (p=0.022). Several factors associated with previous irradiation render those patients more susceptible to aspiration pneumonia, such as thick tenacious saliva, decreased sensation, reduced pharyngeal muscle strength and coordination, and compromised overall medical condition. We speculate that we did not find more pulmonary complications because of the careful patient selection criteria we used. Further evaluation with larger sample sizes is needed to confirm these findings.
More pharyngeal residue was detected during the second MBS study; the reason for this finding is unclear. Due to the retrospective nature of this analysis, a comparison between MBS1 and MBS2 cannot be made because more patients were given solid consistencies during MBS2. This needs to be prospectively evaluated.
An unexpected outcome of this study was the increase in the rate of silent aspiration between MBS1 and MBS2 (27% to 35%). Although the increase was not statistically significant, this finding may be clinically significant in a population at high risk for pulmonary complications. It is interesting that four patients in our study who were sensate to their aspiration on MBS1 were desensate to it on MBS2. Although the reason for this occurrence is unclear, data show that patients may become accustomed to chronic aspiration and become desensate to it over time.15 This finding requires further investigation, but underscores the importance of frequent instrumental swallowing assessment following SCPL. Clinicians who rely on bedside or clinical swallowing examinations in lieu of instrumental testing will not identify the patient who silently aspirates.
Another benefit of the MBS examination is the ability to evaluate the need for, as well as the effectiveness of selected swallowing strategies. Therefore, we strongly recommend a final MBS study for all patients, including those who are successfully using swallowing strategies, to identify patients who may no longer require their use.
Diet modifications, such as thickening liquids, are often the first strategy attempted in response to thin liquid aspiration. Our findings do not support the use of diet modifications alone to prevent aspiration following SCPL. However, when diet modifications were used in combination with other swallowing strategies, they were effective in reducing or eliminating aspiration.
Most clinicians are familiar with the supraglottic swallow maneuver that teaches the patient to volitionally hold his or her breath to intentionally close the airway prior to the swallow to prevent aspiration. While it is sometimes useful, one must be cautious about generalizing the use of a single strategy that benefits patients with intact laryngeal anatomy and physiology to patients with a surgically compromised glottis. The postsurgical defect and reconstruction following SCPL affects structures within the oropharynx, hypopharynx, supraglottis, and glottis, essentially resulting in a complete alteration in the pharyngeal swallow, both anatomically and physiologically. Although neoglottic incompetency was the primary cause of dysphagia in our population, we also identified reduced base of tongue retraction and hyolaryngeal elevation as two other aberrant physiologic findings. Thus, in addition to the supraglottic swallow maneuver, patients also benefited from the use of other swallowing strategies such as the chin tuck posture to narrow the entrance to the airway and increase tongue base opposition to the posterior pharyngeal wall for improved propulsion of the food through the pharynx.16 In our population, the chin tuck posture facilitated arytenoid approximation to the tongue base (CHP, TCHP) or the epiglottis (CHEP) to help protect the airway and improve pharyngeal transit. Thus, our findings also show that patients benefit from the use of a variety of swallowing maneuvers and postures that target all areas of dysfunction.
Finally, our results show little change in the rate of aspiration without the use of compensatory swallowing strategies from MBS1 to MBS2 (100% to 94%, p=0.4500). However, the effectiveness of swallowing strategies in reducing or eliminating aspiration increased from MBS1 to MBS2 (64% to 88%, p=0.0365). Despite the high rate of aspiration, there was a 40% increase (32% to 72%) in the number of patients whose MBS findings indicated an ability to return to full oral nutrition between MBS1 and MBS2 using swallowing strategies. It is likely that a combination of healing, reconstitution of the neoglottic sphincter, swallowing strategy effectiveness, and the ability of the patient to tolerate some degree of continued aspiration allowed patients to return to oral nutrition. The long-term effects of continued aspiration on patient health status require further investigation.
Several studies report higher rates of swallowing recovery with a lower rate of G-tube dependency than our findings demonstrated.17-19 However, the methodology for functional assessment in each is subjectively defined. We evaluated our outcomes based upon strict criteria. In our study, any patient who required a G-tube for partial or full nutrition was not considered able to maintain their nutrition orally. Our practice for removal of G-tubes was based on the findings from instrumental testing and not subjective measures. It is difficult to make a comparison of results between studies whose methodologies are different. Moreover, these studies report results based upon large sample sizes (70 < N < 206). Our sample size was small, and it is possible that a larger sample might yield outcomes similar to published reports.
CONCLUSIONS
We believe that the results of this study offer new and important information that will enhance the quality of patient care and optimize functional recovery in patients after SCPL. Our study represents the first comprehensive analysis of swallowing function in patients who have undergone SCPL that defines the postoperative dysphagia both anatomically and physiologically using scientific and objective instrumental examination. SCPL alters anatomy and physiology and results in severe swallowing dysfunction that should be carefully evaluated and managed by a well-trained speech pathologist experienced in the functional rehabilitation of this patient population.
REFERENCES
- 1.Kotz T, Costello R, Li Y, Posner MR. Swallowing dysfunction after chemoradiation for advanced squamous cell carcinoma of the head and neck. Head Neck. 2004;26:365–72. doi: 10.1002/hed.10385. [DOI] [PubMed] [Google Scholar]
- 2.Mittal BB, Pauloski BR, Haraf DJ. Swallowing dysfunction—preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. Int J Radiation Oncology Biol Phys. 2003;57:1219–30. doi: 10.1016/s0360-3016(03)01454-8. [DOI] [PubMed] [Google Scholar]
- 3.DeSanto LW, Olsen KD, Perry WC, Rohe DE, et al. Quality of life after surgical treatment of cancer of the larynx. Ann Otol Rhinol Laryngol. 1995;104:763–769. doi: 10.1177/000348949510401003. [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre JL, Lartigau E. Preservation of form and function during management of cancer of the larynx and hypopharynx. World J Surg. 2003;27:811–816. doi: 10.1007/s00268-003-7106-5. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein GS, Laccourreye O, Ruiz C. Larynx preservation with supracricoid partial laryngectomy with cricohyoidoepiglottopexy. Ann Otol Rhinol Laryngol. 2002;111:1–7. doi: 10.1177/000348940211100101. [DOI] [PubMed] [Google Scholar]
- 6.Laccourreye H, Laccourreye O, Weinstein G, et al. Supracricoid laryngectomy with cricohyoidoepiglottopexy: a partial laryngeal procedure for glottic carcinoma. Ann Otol Rhinol Laryngol. 1990;99:421–426. doi: 10.1177/000348949009900601. [DOI] [PubMed] [Google Scholar]
- 7.Laccourreye O, Brasnu D, Perie S, et al. Supracricoid partial laryngectomies in the elderly: mortality, complications, and functional outcome. Laryngoscope. 1998;108:237–242. doi: 10.1097/00005537-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Lima RA, Freitas EQ, Kligerman J, et al. Supracricoid laryngectomy with CHEP: functional results and outcome. Otolaryngol Head Neck Surg. 2001;124:258–260. doi: 10.1067/mhn.2001.113138. [DOI] [PubMed] [Google Scholar]
- 9.Makeieff M, Venegoni D, Mercante G, et al. Supracricoid partial laryngectomies after failure of radiation therapy. Laryngoscope. 2005;115:353–357. doi: 10.1097/01.mlg.0000154751.86431.41. [DOI] [PubMed] [Google Scholar]
- 10.Naudo P, Laccourreye O, Weinstein G, et al. Functional outcome and prognosis factors after supracricoid partial laryngectomy with cricohyoidopexy. Ann Otol Rhinol Laryngol. 1997;106:291–296. doi: 10.1177/000348949710600405. [DOI] [PubMed] [Google Scholar]
- 11.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2nd ed. Pro-Ed; Austin, TX: 1998. pp. 168–80. [Google Scholar]
- 12.Holsinger FC, Laccourreye O, Weinstein GS, et al. Technical refinements in the supracricoid partial laryngectomy to optimize functional outcomes. J Am Coll Surg. 2005;201:809–820. doi: 10.1016/j.jamcollsurg.2005.06.260. [DOI] [PubMed] [Google Scholar]
- 13.Kies M, Lewin J, Diaz E, et al. Definitive treatment of intermediate stage laryngeal squamous cell cancer with chemotherapy. Abstract. J Clin Onc, 2004 American Society of Clinical Oncology Annual Meeting Proceedings. 2004;22:14S, 5533. [Google Scholar]
- 14.Laccourreye O, Weinstein G, Naudo P, et al. Supracricoid partial laryngectomy after failed laryngeal radiation therapy. Laryngoscope. 1996;106(4):495–498. doi: 10.1097/00005537-199604000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Markkanen-Leppanen M, Isotalo E, Makitie AA, et al. Swallowing after free-flap reconstruction in patients with oral and pharyngeal cancer. Oral Oncol. 2006;42:501–509. doi: 10.1016/j.oraloncology.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2nd ed. Pro-Ed; Austin, TX: 1998. p. 94. [Google Scholar]
- 17.de Vincentiis M, Minni Gallo A. Supracricoid laryngectomy with cricohyoidopexy (CHP) in the treatment of laryngeal cancer: a functional and oncologic experience. Laryngoscope. 1996;106(9 Pt 1):1108–1114. doi: 10.1097/00005537-199609000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Laudadio P, Presutti L, Dall’olio D, Cunsolo E, Consalici R, Amorosa L, Cancellieri A, Bocciolini C. Supracricoid laryngectomies: long-term oncological and functional results. Acta Otolaryngol. 2006;126(6):640–649. doi: 10.1080/00016480500469024. [DOI] [PubMed] [Google Scholar]
- 19.Pellini R, Manciocco V, Spriano G. Functional outcome of supracricoid partial laryngectomy with cricohyoidopexy: radiation failure vs previously untreated cases. Arch Otolaryngol Head Neck Surg. 2006;132(11):1221–1225. doi: 10.1001/archotol.132.11.1221. [DOI] [PubMed] [Google Scholar]