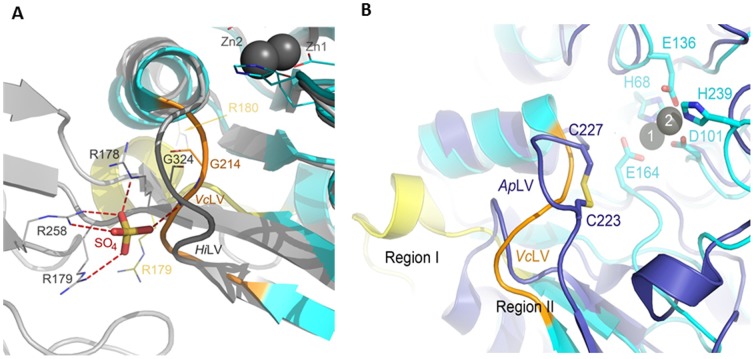
Figure 6. The Role of the Dimerization Domain in the Stabilization of Loop V in WT-HiDapE.
A) Superimposition of the WT-HiDapE (gray) and VcDapET (cyan) structures is shown. Loop V of WT-HiDapE and VcDapET is labeled as HiLV and VcLV, respectively. WT-HiDapE residues interacting with the sulfate ion (stick model) are shown as gray lines. Corresponding residues in VcDapET (except for R258 that is absent in the deletion mutant) are shown as yellow (R179 and R180) and orange (G214) lines. B) Specific orientation of the active site loop V in VcDapET and the corresponding loop in AAP. Overlay of the VcDapET (cyan) and AAP (purple) structures is shown. The AAP loop and VcDapET loop V are labeled as ApLV and VcLV, respectively. Stabilization of loop V in AAP by a disulfide bridge is indicated where Cys223 and Cys227 of AAP and the residues involved in zinc-binding in VcDapET are shown as sticks. Zinc ions of VcDapET are shown as black spheres. Zinc-bound ethylene glycol was omitted for clarity.