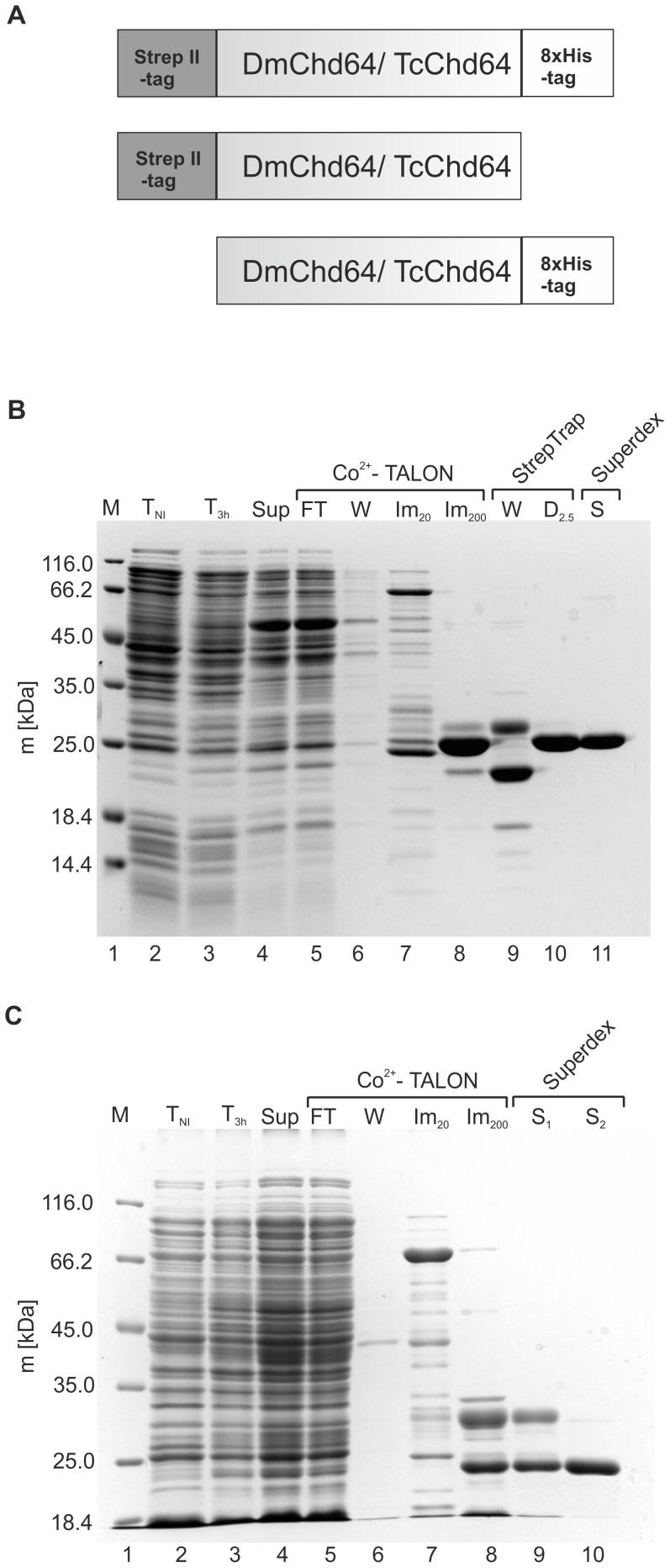
Figure 3. Schematic illustration and purification of recombinant DmChd64 and TcChd64.
(A) Schematic illustration of recombinant DmChd64 and TcChd64. Recombinant constructs for DmChd64 and TcChd64 were prepared. DmChd64/TcChd64was N-terminally tagged with the Strep II-tag, and C-terminally tagged with the 8×His-tag. DmChd64/TcChd64 was N-terminally tagged with the Strep II-tag and DmChd64/TcChd64 was C-terminally tagged with the 8×His-tag. (B) Coomasie Brilliant Blue R-250-stained SDS-PAGE analysis of the expression and purification procedure of DmChd64. Lane 1, molecular mass standards; lane 2 and 3, whole cell extracts from bacterial cells containing the pQE80L-SXH plasmid with the cDNA insert (pQE80L-SXH/DmChd64) harvested before (TNI) and 3 h after expression induction with IPTG (T3 h); lane 4, soluble protein fractions obtained after cell lysis (Sup); lane 5, the flow-through (FT) fraction obtained from the Co2+-TALON column; lane 6, protein fractions containing impurities washed (W) from the Co2+-TALON column using buffer A; lane 7, protein fractions containing impurities eluted during the imidazole gradient sub-step using buffer A and B solutions containing 20 mM imidazole (Im20); lane 8, pooled fractions eluted from Co2+-TALON resin with buffer B containing 200 mM imidazole (Im200); lane 9, protein fractions containing impurities washed (W) from the Strep-Trap HP column using buffer C; lane 10, pooled fractions eluted from the Strep-TrapHP column with buffer D containing 2.5 mM desthiobiotin (D2.5); lane 11, pooled fractions of DmChd64 from the Superdex 200 10/300 GL column (S). (C) Coomasie Brilliant Blue R-250-stained SDS-PAGE analysis of the expression and purification procedure of TcChd64. Lane 1, molecular mass standards; lane 2 and 3, whole cell extracts from bacterial cells containing the pQE80L-XH plasmid with the cDNA insert (pQE80L-XH/TcChd64) harvested before (TNI) and 3 h after expression induction with IPTG (T3 h); lane 4, soluble protein fractions obtained after cell lysis (Sup); lane 5, the flow-through (FT) fraction obtained from the Co2+-TALON column; lane 6, protein fractions containing impurities washed (W) from the Co2+-TALON column using buffer E; lane 7, protein fractions containing impurities eluted during the imidazole gradient sub-step using buffer E and F solutions containing 20 mM imidazole (Im20); lane 8, pooled fractions eluted from the Co2+-TALON resin with buffer F containing 200 mM imidazole (Im200); lane 9, protein fractions containing impurities from the first peak from tandem connected Superdex 75 10/300 GL columns (S1); lane 10, pooled fractions of TcChd64 from the two tandem Superdex 75 10/300 GL columns (S2).