Abstract
Metastasis is a multistep process involving modification of morphology to suit migration, reduction of tumor cell adhesion to the extracellular matrix, increase of cell mobility, tumor cell resistance to anoikis, and other steps. MicroRNAs are well-suited to regulate tumor metastasis due to their capacity to repress numerous target genes in a coordinated manner, thereby enabling their intervention at multiple steps of the invasion-metastasis cascade. In this study, we identified a microRNA exemplifying these attributes, miR-124, whose expression was reduced in aggressive MDA-MB-231 and SK-3rd breast cancer cells. Downregulation of miR-124 expression in highly aggressive breast cancer cells contributed in part to DNA hypermethylation around the promoters of the three genes encoding miR-124. Ectopic expression of miR-124 in MDA-MB-231 cells suppressed metastasis-related traits including formation of spindle-like morphology, migratory capacity, adhesion to fibronectin, and anoikis. These findings indicate that miR-124 suppresses multiple steps of metastasis by diverse mechanisms in breast cancer cells and suggest a potential application of miR-124 in breast cancer treatment.
Keywords: miR-124, breast cancer, metastasis, pro-metastasis gene
Metastasis is responsible for >90% of cancer-related deaths[1]. Metastasis is a multistep process involving cell detachment from the primary tumor, extravasation, and, finally, invasion to the secondary site. Many researches have been focused on identifying the critical regulators of the metastatic process. These regulatory molecules include both proteins and microRNAs (miRNAs)[2],[3].
miRNAs are small non-coding RNA molecules that suppress gene expression by interacting with the 3′-untranslated regions (UTRs) of target mRNAs. These interactions may result in either inhibited translation or degradation of targeted mRNAs[4]. An individual miRNA can regulate dozens of distinct mRNAs, involving different steps for regulating the metastasis of breast cancer cells[5], indicating the important role and multiple functions of miRNAs in control of tumor progression.
The regulatory mechanism of miRNA expression has been documented at the transcriptional and post-transcriptional processing levels. In cancer, transcriptional silencing due to epigenetic pathways is a significant alteration[6]. Hypermethylation of gene promoters is a frequent mechanism of miRNA silencing at the transcriptional level[7]. The facts that miRNA expression occurs in a tissue-specific or developmental stage-specific manner and that some miRNAs are imprinted[8] support the hypothesis that DNA methylation may regulate miRNA expression, as indicated in recent studies[9]–[11]. Therefore, it is conceivable that DNA methylation regulates miRNA expression during tumor progression.
miR-124, which is highly expressed in the central nervous system, was found to be epigenetically silenced in a variety of tumor cells[9]–[12] and modulate the proliferation of tumor cells by targeting Cyclin-dependent kinase 6 (CDK6) [13]–[15]. More recently, Hunt et al.[16] reported that miR-124 could also suppress the motility of oral squamous cell carcinoma by targeting integrin-β1 (ITGB1). Additionally, Zheng et al.[17] found that miR-124 modulated hepatocellular carcinoma cell aggressiveness by repressing the expression of Rho-associated coiled-coil containing protein kinase 2 (ROCK2) and enhancer of zeste homolog 2 (EZH2). These data suggest a potential tumor suppressive function for miR-124. To date, however, the role of miR-124 in breast cancer and the molecular mechanisms by which miR-124 expression is regulated remain largely unknown.
In this study, we report that dysregulation of miR-124 was correlated with metastatic potential. Ectopic expression of miR-124 in breast cancer cells suppressed multiple steps of metastasis. More specifically, we provide evidence that miR-124 directly suppressed multiple pro-metastasis targets, including connective tissue growth factor (CTGF), ras homolog gene family member G (RhoG), ITGB1, and ROCK1. In addition, DNA hypermethylation of CpG islands in the promoters of MIR124-1, MIR124-2, and MIR124-3 might contribute in part to the down-regulated expression of miR-124 in highly aggressive breast cancer cell lines. Collectively, our results show that miR-124, a pleiotropically acting miRNA, suppressed multiple steps of breast cancer metastasis and suggest that miR-124 may be a new target for breast cancer therapy.
Materials and Methods
Cell lines and culture
The breast cancer cell lines BT474, MDA-MB-231, MCF-7, and MDA-MB-436 were obtained from the American Type Culture Collection (ATCC; Manassas, VA). SK-3rd was previously established by consecutively passaging breast cancer cell line SKBR3 in NOD/SCID mice under the pressure of chemotherapy[18]. BT474, MDA-MB-231, MDA-MB-436, and SK-3rd cells were maintained in DMEM (Gibco, USA) complete medium (90% DMEM and 10% FBS). For MCF-7 cells, additional insulin was added to the DMEM complete medium. For demethylation, cells cultured in DMEM complete medium were treated with 5′-aza-2- deoxycytidine (AZA) (Sigma, USA) at a concentration of 2 µmol/L or DMSO for 72 h.
Genomic DNA isolation and bisulfite DNA sequencing PCR (BSP) analysis
Genomic DNAs were isolated from cells using the DNeasy Tissue Kit (Qiagen, USA). DNA samples were treated with sodium bisulfite to convert cytosine to uracil using the Methyl Detector™ Bisulfite Modification Kit (Active Motif, North America) according to the manufacturer's instructions.
For BSP, a 1 µL aliquot of sodium bisulfite-treated DNA was amplified by PCR with commonly used primers for methylated and unmethylated DNA sequences. The PCR products were cloned into the pGEM-T Easy vector (Promega, USA), and 10 clones from each sample were sequenced to determine the methylation status of each CpG site. BSP primers for MIR-124-1, MIR-124-2, and MIR-124-3 were designed according to previously validated oligonucleotides[10] and synthesized commercially (Invitrogen, USA).
Oligonucleotide transfection
miR-124 mimics and negative control were purchased from GenePharma (Shanghai, China). Small interfering RNAs (siRNAs) targeting human CTGF[19], ITGB1[20],[21], RhoG[22], and ROCK1[23],[24] were designed according to previously validated oligonucleotides and synthesized by GenePharma. Oligonucleotide transfection was performed with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer's instructions.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with TRIzol (Invitrogen, USA) according to the manufacturer's instructions. cDNA was synthesized with MLV transcriptase kit (Invitrogen, USA). Real-time PCR was performed with LightCycler480 System using SYBR Premix Ex Taq kit (TaKaRa, Japan). The silencing effects on CTGF, ITGB1, RhoG, and ROCK1 were evaluated using qRT-PCR. The sequences of qRT-PCR primers for CTGF[19], ITGB1[25], RhoG[26], and ROCK1[27] were validated previously and synthesized by Invitrogen. The mature form of miRNAs was detected using the miRNA qPCR Quantitation Assay according to the manufacturer's instructions (GenePharma, China). The U6 small nuclear RNA purchased from GenePharma was used as an internal control.
Western blotting
Western blot analysis was performed as described previously[28]. Briefly, cells were lysed in MCLB [50 mmol/L Tris-HCI, pH 8.0, 100 mmol/L NaCl, 5 mmol/L EDTA, 0.5% Nonidet P-40, 2 mmol/L dithiothreitol (DTT), and 2 mmol/L phenylmethylsulfonyl fluoride (PMSF)] and clarified lysates were resolved by SDS-PAGE and transferred to nitrocellulose membranes for Western blotting. GAPDH antibody (PeproTech, USA) and ROCK1 antibody (Santa Cruz Biotechnology, USA) were used in accordance with the manufacturer's instructions.
Luciferase reporter assay
Putative miR-124–binding sites in the 3′-UTR of the ROCK1, CTGF, RhoG, and ITGB1 mRNAs were cloned into pMIR-REPORT vector (Ambion, USA). Mutations were detected using QuikChange Site-Directed Mutagenesis Kit (Stratagene, USA) according to the manufacturer's instructions. The primers used for construction of luciferase reporters and mutations of miR-124–binding sites are listed in Table 1.
Table 1. Primers for CTGF, RhoG, ITGB1, and ROCK1 luciferase reporter constructures.
| Gene | Forward primer sequence | Reverse primer sequence |
| CTGF | 5′ -CGACTAGTGCCAGAGAGTGAGAGACATTAAC-3′ | 5′-TGAACGATCAGACAAGCTTTAC-3′ |
| RhoG | 5′-TTACTAGTCCCTGGCACTTGGCTTGGA-3′ | 5′-TAGAAGCTTGAGTCAGTCAGCAAATGCGT-3′ |
| ITGB1 | 5′ -TAGAGCTCCCGTGCAAATCCCACAACA-3′ | 5′-ATACGCGTTACATCAGAGTCAAGACATCCG-3′ |
| ROCK1 | 5′-TTGAGCTCGTGCCCTGTGGAATCGTG-3′ | 5′-TCACGCGTTTATGTTGGTGCAACCTTCTA-3′ |
| CTGF mutant | 5′-TTAAAGTTGTTTCTCCGTCTTTATTnTG-3′ | 5′-CAAAAATAAAGACGGAGAAACAACTTTAA-3′ |
| RhoG mutant | ||
| Site-1 | 5′-CCCACCAGTTATACATAGGTGCCTTGTCC-3′ | 5′-GGACAAGGCACCTATGTATAACTGGTGGG-3′ |
| Site-2 | 5′-TCCGCCTCAGCTATACATAAAGGACTAATTC-3′ | 5′-GAATTAGTCCTTTATGTATAGCTGAGGCGGA-3′ |
| Site-3 | 5′-CTTTTTCTCTGAATACATATTTCTCCTTAAG-3′ | 5′-CTTAAGGAGAAATATGTATTCAGAGAAAAAG-3′ |
| ITGB1 mutant | ||
| Site-1 | 5′-TAAGGTCACATTCTCCGTCTTTGACCTTTTC-3′ | 5′-GAAAAGGTCAAAGACGGAGAATGTGACCTTA-3′ |
| Site-2 | 5′-ACATTCTTGTTTTAACTCCGTCTAGTTTTAACAG-3′ | 5′-CTGTTAAAACTAGACGGAGTTAAAACAAGAATGT-3′ |
| ROCK1 mutant | 5′-ATTGTCCTTTTACTCCGTCAATTTGAGAT-3′ | 5′-ATCTCAAATTGACGGAGTAAAAGGACAAT-3′ |
CTGF, connective tissue growth factor; RhoG, ras homolog gene family member G; ITGB1, integrin-β1; ROCK1, Rho-associated coiled-coil containing protein kinase 1.
The firefly luciferase constructs were co-transfected with a control Renilla luciferase vector pRL-TK (Promega, USA) into MBA-MD-231 cells in the presence of either miR-124 mimics or negative control. A dual luciferase assay (Promega, USA) was performed 24 h after transfection. The experiments were performed independently in triplicate.
Wound healing and Transwell assays
Cell motility was assessed by measuring the movement of cells into a scraped, acellular area created by a 200 µL pipette tip. The spread of wound closure was observed after 24 h and 48 h and photographed under a microscope.
Migration assays were carried out in modified Boyden chambers with 8 µm pore filter inserts in 24-well plates (BD Transduction, USA). Briefly, 1 × 10s cells suspended in serum-free DMEM were added to the upper chamber of the insert in each well of a 24-well culture plate. FBS was added to the lower chamber as a chemoattractant. After 8 h, the non-filitered cells were gently removed with a cotton swab. Filtered cells located on the lower side of the chamber were stained with crystal violet, air dried, and photographed.
Anoikis assay
MBA-MD-231 cells were suspended in growth medium at a density of 5 × 105 cells/mL and plated on ultra-low attached anoikis plates for 72 h. Cells were then washed with 1× PBS and stained using the Annexin V-FITC Apoptosis Detection Kit (KeyGEN Biotech, Nanjing, China) according to the manufacturer's instructions. Briefly, FITC-labeled annexin V and propidium iodide were added to the cells, which were incubated in the dark at room temperature for 15 min and then analyzed using flow Cytometry. Each experiment was carried out at least three times.
Cell adhesion assay
MDA-MB-231 cells (2 × 105 cells/100 µL) were transfected with miR-124 mimics, negative control, or mock and were seeded to 24-well plates coated with fibronectin at 20 µg/mL and incubated for 1 h. The cells were fixed with 4% paraformaldehyde. Separated cells were washed off with PBS. Cells that adhered to the substrate were stained with crystal violet and observed under a microscope.
Results
miR-124 expression is attenuated in metastatic breast cancer cell lines
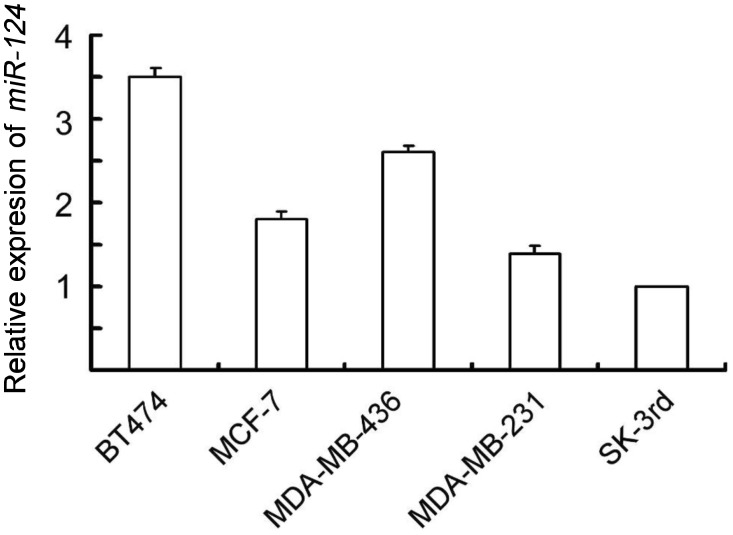
To explore the potential role of miR-124 in breast cancer, we examined the expression level of miR-124 in a panel of breast cancer cell lines with distinct metastatic capacity. As shown in Figure 1, the expression level of miR-124 was attenuated in aggressive human breast cancer cells, MDA-MB-231, and SK-3rd, compared to non-metastatic BT474 cells. The decreased expression level suggests an inhibitory effect of miR-124 on metastasis of breast cancer cells.
Figure 1. miR-124 expression in breast cancer cell lines.
Expression levels of miR-124 were examined by real-time polymerase chain reaction (PCR) in 5 breast cancer cell lines. Columns, mean of three independent experiments; bars, standard deviation (SD).
miR-124 expression suppresses metastasis-relevant traits in vitro
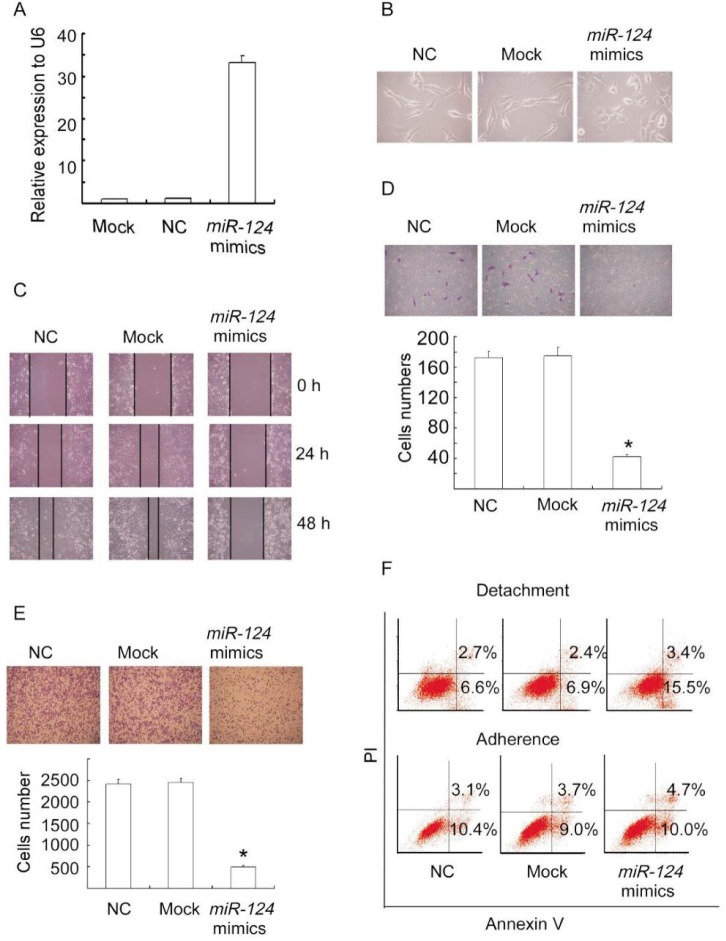
Given the inverse correlation between miR-124 level and aggressive phenotype, we assessed the potential anti-metastatic role of miR-124 in breast cancer cells. We transiently transfected MDA-MB-231 cells with miR-124 mimics, negative control, or mock (untreated cells) and examined metastasis-relevant traits in vitro. Overexpression of miR-124 mimics in MDA-MB-231 cells was detected by qRT-PCR (Figure 2A). Ectopic miR-124 expression resulted in a switch in cellular shape from spindle-shaped to round (Figure 2B), a phenotype that presumably indicated less invasiveness.
Figure 2. miR-124 expression suppresses metastasis-relevant traits in vitro.
A, overexpression of miR-124 in MDA-MB-231 cells was detected by quantitative real-time PCR (qRT-PCR). MDA-MB-231 cells were transfected with miR-124 mimics, negative control (NC), or mock for 48 h and the RNAs were extracted. B, the morphology of MDA-MB-231 cells changed from spindle-shaped to round as observed under a microscope after transfection with miR-124 mimics, negative control, or mock for 48 h. C, the wound-healing assay shows decreased cell motilities in miR-124 ectopically expressed MDA-MB-231 cells. MDA-MB-231 cells transfected with miR-124 mimics, negative control, or mock were cultured with serum-free medium for 24 h and scraped to created acellular area. The spread of wound closure was observed 24 h and 48 h after scrape and photographed under a microscope. D, ectopic expression of miR-124 suppresses the migratory capacity of MDA-MB-231 cells. MDA-MB-231 cells transfected with miR-124 mimics, negative control, or mock for 48 h were placed in serum-free medium and added to the upper chamber of transwell plates. Medium containing 10% serum was added to the lower chamber as a chemoattractant. The migratory capacity was assessed by calculating the filtered cells. Columns, mean of three independent experiments; bars, SD. *P < 0.01, vs. negative control and mock cells. E, ectopic expression of miR-124 reduces the adhesion of MDA-MB-231 cells to fibronectin. MDA-MB-231 cells transfected with miR-124 mimics, negative control, or mock for 48 h were detached from culture dishes with trypsin and suspended in serum-free medium. The suspended cells were seeded to 24-well plates coated with fibronectin for 30 min and then observed under a microscope. Columns, mean of three independent experiments; bars, SD. *P < 0.01, vs. control and mock cells. F, ectopic expression of miR-124 increases the sensitivity of MDA-MB-231 cells to anoikis. MDA-MB-231 cells transfected with miR-124 mimics, negative control, or mock for 24 h were detached from culture dishes with trypsin and seeded to anoikis plates for another 72 h. Apoptotic cells were evaluated by staining with FITC-annexin V and propidium iodide (PI) and analyzed with FACS.
To determine whether miR-124 expression suppresses the migratory capacity of MDA-MB-231 cells, a wound-healing assay was used. Duplicate experiments consistently showed decreased migratory capacity of MDA-MB-231 cells expressing miR-124 mimics compared to MDA-MB-231 control cells and mock cells at 24 and 48 h (Figure 2C). A migration chamber assay was also used to further assess the migratory capacity of MDA-MB-231 cells overexpressing miR-124. Ectopic expression of miR-124 reduced migrated cells by 4 folds compared to negative control and mock (Figure 2D).
Because adhesive capacity is one of the important traits contributing to metastasis, we examined the adhesion of MDA-MB-231 cells overexpressing miR-124 to fibronectin. As expected, miR-124 expression dramatically suppressed the adhesive capacity of MDA-MB-231 cells (Figure 2E).
In addition, we studied whether miR-124 expression reduced the resistance of MDA-MB-231 cells to anoikis, another important process contributing to metastasis of cancer cells. As shown in Figure 2F, MDA-MB-231 cells overexpressing miR-124 were more sensitive to anoikis than control and mock cells. Together, these results indicate that miR-124 suppresses multiple steps of metastasis of breast cancer cells.
miR-124 directly regulates a cohort of prometastatic genes
To explore the targets directly regulated by miR-124, we used two algorithms that predict the mRNA targets of a miRNA-PicTar[29] and TargetScan[30]. Based on the representation of miR-124 sites in their 3′-UTRs, >1000 mRNAs were predicted to be regulated by miR-124. Gene Ontology revealed that these targets included a large number of genes encoding proteins with roles in motility-related processes, such as cell adhesion, cytoskeletal remodeling, and cell polarity (data not shown).
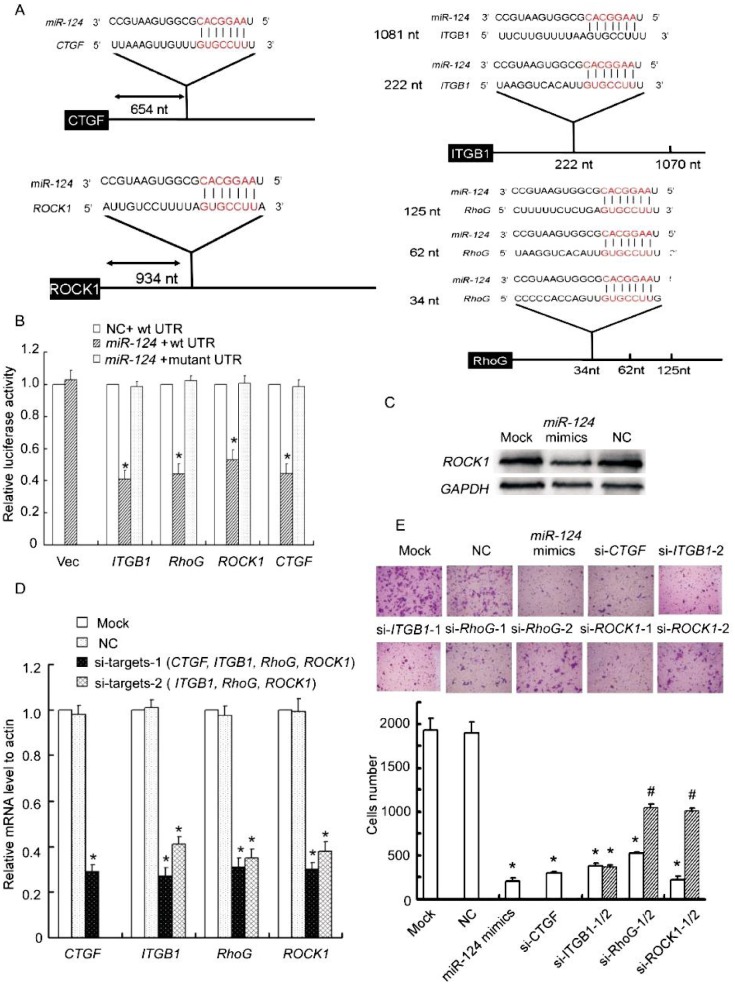
Guided by this Gene Ontology analysis, we cloned the 3′-UTRs of 4 putative miR-124 targets from these overrepresented categories, including CTGF, RhoG, ROCK1, and ITGB1 (a well-validated target in oral squamous cell carcinoma) into a luciferase plasmid (Figure 3A). The fluorescence activities of 3′-UTR reporters of IPTG1, RhoG, and CTGF were suppressed by miR-124 mimics by more than 2 folds as compared to negative control, whereas that of ROCK1 was reduced to 55%; mutations of the putative miR-124–binding sites in the four 3′-UTRs abrogated response to miR-124 mimics (Figure 3B). To further confirm that miR-124 directly targets these genes, we examined whether miR-124 expression reduced the endogenous protein level of ROCK1, the target least suppressed by miR-124 in the luciferase reporter assay. As shown in Figure 3C, miR-124 expression obviously decreased the protein level of ROCK1 compared to negative control and mock.
Figure 3. CTGF, RhoG, ITGB1, and ROCK1 are targets of miR-124.
A, schematic illustration of the predicted miR-124–binding sites in the 3′-UTRs of CTGF, RhoG, ITGB1, and ROCK1. B, miR-124 significantly reduces the luciferase activities of CTGF, RhoG, ITGB1, or ROCK1. miR reporter constructs containing wild-type and mutated 3′ -UTRs of the 4 putative target genes were co-transfected with miR-124 mimics or negative control into MDA-MB-231 cells and incubated for 24 h. Relative repression of firefly luciferase expression was standardized to a transfection control. Columns, mean of three independent experiments; bars, SD; *P < 0.01, vs. negative control. C, ectopic expression of miR-124 decreases endogenous levels of ROCK1. MDA-MB-231 cells were transfected with miR-124 mimics, negative control, or mock for 72 h. ROCK1 expression was assessed by Western blotting. D, siRNA transfection potently reduces target mRNA levels. MDA-MB-231 cells were transfected with siRNAs against CTGF, ITGB1, RhoG, ROCK1, negative control, or mock for 48 h. The target mRNA levels were evaluated by realtime quantitative PCR. E, siRNAs against CTGF, ITGB1, RhoG, or ROCK1 reduce the migratory ability of MDA-MB-231 cells. The migration of siRNA-transfected MDA-MB-231 cells was assessed using transwell assays. Columns, mean of three independent experiments; bars, SD. *P < 0.001, # P < 0.01, vs. negative control and mock cells.
To assess the functional contributions of these miR-124 targets to aggressive phenotypes, we examined if their inhibition affected the migration of MDA-MB-231 cells using transwell assays. Transfection with siRNAs potently reduced mRNA levels of CTGF, ITGB1, RhoG, and ROCK1 (Figure 3D). As expected, siRNAs against CTGF, ITGB1, RhoG, and ROCK1 reduced filtered MDA-MB-231 cells markedly (Figure 3E).
Collectively, these observations demonstrated that miR-124 directly regulates a cohort of pro-metastatic genes including CTGF, ITGB1, RhoG, and ROCK1.
Transcriptional down-regulation of miR-124 in aggressive breast cancer cells contributes in part to DNA hypermethylation in the promoters of the genes encoding miR-124
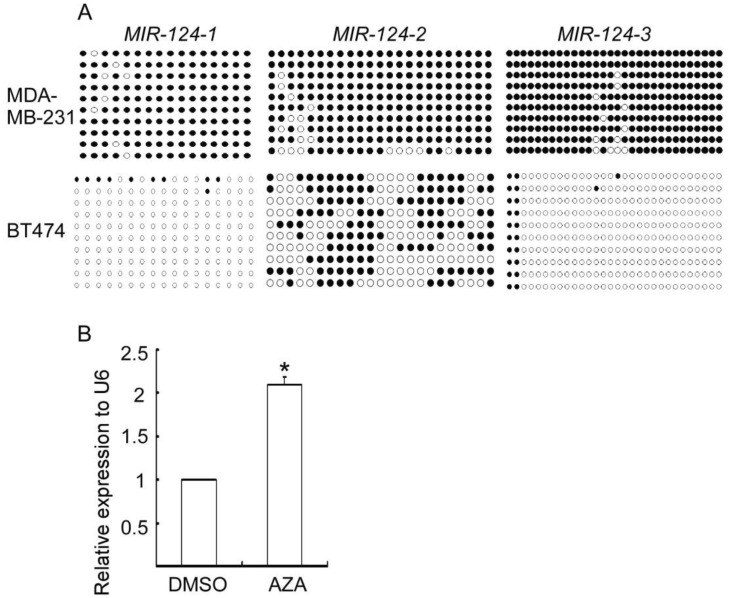
miR-124 was reported to be down-regulated by DNA hypermethylation in its promoters in several types of cancer[9]. We hypothesized that DNA hypermethylation resulted in down-regulated miR-124 level in aggressive breast cancer cells. Thus, we analyzed the methylation status of the putative promoters of its three genes (MIR-124-1, MIR-124-2, and MIR-124-3) in MDA-MB-231 and BT474 cells, which are highly aggressive and non-metastatic breast cancer cells, respectively, by bisulfite sequencing PCR (BSP) sequence assay. As shown in Figure 4A, the promoters of MIR-124-1, MIR-124-2, and MIR-124-3 were heavily methylated in aggressive MDA-MB-231 cells but only methylated at low levels in non-metastatic BT474 cells. More importantly, treatment of MDA-MB-231 cells with the DNA demethylating agent AZA partially restored miR-124 expression (Figure 4B). These results indicate that DNA hypermethylation contributes at least in part to the decreased miR-124 expression in aggressive MDA-MB-231 cells.
Figure 4. DNA hypermethylation contributes in part to the down-regulated expression of miR-124 in MDA-MB-231 cells.
A, the methylation status of CpG islands around MIR124-1, MIR124-2, and MIR124-3 were analyzed by bisulfite sequencing. Closed circle, methylated CpG site; open circle, unmethylated CpG site. B, compared to control, expression of miR-124 is up-regulated in MDA-MB-231 cells treated with demethylation agent 5-aza-2-deoxycytidine (AZA).
Discussion
miRNAs can modulate a wide variety of targets, which endowed the supposition that a single miRNA might regulate cancer progression in multiple steps by targeting numerous genes. In this study, we demonstrate that a single human miRNA, miR-124, can concomitantly repress multiple pro-metastatic targets and thereby to inhibit several distinct steps of the invasion-metastasis cascade in breast cancer cells. In addition, low-level expression of miR-124 and high-level methylation of MIR-124 promoters were observed in highly metastatic breast cancer cells. Moreover, treatment of MDA-MB-231 cells with demethylation agent AZA partially restored miR-124 expression, indicating that DNA hypermethylation plays an important role in down-regulation of miR-124 expression in highly aggressive breast cancer cells.
Metastasis is a multistep process. Therefore, identifying factors that modulate several steps of metastasis and understanding the underlying molecular mechanisms involved in tumor metastasis progression are critical[5]. Tumor cells undergoing mesenchymal-to-epithelial transition (MET) have been reported to exhibit a switch from a spindle-like to a round morphology and to have reduced metastatic capacity. Our results show that MDA-MB-231 cells with ectopic expression of miR-124 became rounder than control cells. In addition, miR-124 expression in MDA-MB-231 cells resulted in down-regulation of the protein level of vimentin, a mesenchymal marker. However, the protein level of epithelial marker E-cadherin was not visibly changed upon miR-124 expression (data not shown). These observations suggest that ectopic expression of miR-124 might regulate MET in part in breast cancer cells. In addition, cell motility, migratory and adhesive ability, and resistance to anoikis are important factors contributing to cancer cell metastasis to distant sites. In the current study, we evaluated the effects of miR-124 expression on these crucial steps of metastasis and found that reintroduction of miR-124 into MDA-MB-231 cells reduced cell mobility and migration, repressed cell adhesion to the extracellular matrix, and increased cell sensitivity to anoikis. These observations indicate that miR-124 is a powerful metastasis suppressor.
Subsequently, we assessed CTGF, ITGB, RhoG, and ROCK1 as potential functional targets of miR-124. Our results show that miR-124 bound the complementary sites in the 3′-UTRs of the CTGF, ITGB1, RhoG, and ROCK1 genes. Luciferase reporter assay indicated that miR-124 suppressed the reporter fluorescence activity delivered 3′-UTRs of these genes. In addition, we analyzed the effects of miR-124 expression on the mRNA levels of these genes in the GEO Profiles Database (http://www.ncbi.nlm.nih.gov/geoprofiles/). The microarray data showed that the mRNA levels of the four genes were dramatically down-regulated in HepG2 hepatocellular carcinoma cells with ectopic expression of miR-124 compared to negative control. These observations provide the first evidence, to our knowledge, that miR-124 mechanistically acts via the regulation of CTGF, ITGB1, RhoG, and ROCK1. CTGF, RhoG, ITGB1, and/or ROCK1 are reportedly up-regulated in several types of human cancer, including breast cancer, and overexpression of these proteins is positively correlated with tumor metastasis and/or poor prognosis[31]–[34]. This is consistent with our findings that down-regulation of miR-124 is correlated with highly aggressive breast cancer. Furthermore, miR-124 has been found to modulate hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2[17]. Thus, our findings, together with the work of other groups, demonstrate that miR-124 may target multiple proteins that function spatiotemporally or in cooperation with different cellular processes.
Previous studies have described effects of specific miRNAs on local invasion, an early stage of the invasion-metastasis cascade. The present work demonstrates that miRNAs can also influence later steps of metastasis and that an individual miRNA can intervene at multiple distinct stages of the invasion-metastasis cascade. miR-124 regulates the motility, adhesion to ECM, and the intraluminal survival of breast cancer cells.
Collectively, the findings in the present study carry significant implications regarding our understanding of the pathogenesis of high-grade breast cancer. Our data suggest that down-regulation of miR-124 expression is correlated with aggressiveness in breast cancer cell lines. Ectopic expression of miR-124 suppresses multiple distinct steps of the invasion-metastasis cascade in vitro. As distant metastases are responsible for patient mortality, miR-124, with the ability to impede metastasis, may prove to be clinically useful.
Acknowledgments
This work was supported by grants from 973 Projects from Ministry of Science and Technology of China (No. 2010CB912800, 2011CB504203, 2009CB521706), A3 program of Natural Science Foundation of China (30921140312), the Natural Science Foundation of China (No. 30831160515, 30830110, 30973396, 81102023), Clinical Key Project of Public Health Administration of China, and Natural Science Foundation of Guangdong Province (No. 8251008901000011, S2011040004481), Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, Sun Yat-sen University (No. KLB09001).
References
- 1.Gupta GP, Massague J. Cancer metastasis: building a framework [J] Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Nicoloso MS, Spizzo R, Shimizu M, et al. MicroRNAs—the micro steering wheel of tumour metastases [J] Nat Rev Cancer. 2009;9(4):293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 3.Ma L, Weinberg RA. Micromanagers of malignancy: role of microRNAs in regulating metastasis [J] Trends Genet. 2008;24(9):448–456. doi: 10.1016/j.tig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP, et al. MicroRNAs: genomics, biogenesis, mechanism, and function [J] Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Valastyan S, Reinhardt F, Benaich N, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis [J] Cell. 2009;137(6):1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? [J] Nat Rev Cancer. 2006;6(2):107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 7.Khraiwesh B, Arif MA, Seumel GI, et al. Transcriptional control of gene expression by microRNAs [J] Cell. 2010;140(1):111–122. doi: 10.1016/j.cell.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Seitz H, Youngson N, Lin SP, et al. Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene [J] Nat Genet. 2003;34(3):261–262. doi: 10.1038/ng1171. [DOI] [PubMed] [Google Scholar]
- 9.Lujambio A, Ropero S, Ballestar E, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells [J] Cancer Res. 2007;67(4):1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 10.Ando T, Yoshida T, Enomoto S, et al. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect [J] Int J Cancer. 2009;124(10):2367–2374. doi: 10.1002/ijc.24219. [DOI] [PubMed] [Google Scholar]
- 11.Agirre X, Vilas-Zornoza A, Jimenez-Velasco A, et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia [J] Cancer Res. 2009;69(10):4443–4453. doi: 10.1158/0008-5472.CAN-08-4025. [DOI] [PubMed] [Google Scholar]
- 12.Wilting SM, van Boerdonk RA, Henken FE, et al. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer [J] Mol Cancer. 2010;9:167. doi: 10.1186/1476-4598-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong KY, So CC, Loong F, et al. Epigenetic inactivation of the miR-124-1 in haematological malignancies [J] PLoS One. 2011;6(4):e19027. doi: 10.1371/journal.pone.0019027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta M, Kozaki KI, Tanaka S, et al. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma [J] Carcinogenesis. 2010;31(5):766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]
- 15.Pierson J, Hostager B, Fan R, et al. Regulation of cyclin dependent kinase 6 by microRNA 124 in medulloblastoma [J] J Neurooncol. 2008;90(1):1–7. doi: 10.1007/s11060-008-9624-3. [DOI] [PubMed] [Google Scholar]
- 16.Hunt S, Jones AV, Hinsley EE, et al. MicroRNA-124 suppresses oral squamous cell carcinoma motility by targeting ITGB1 [J] FEBS Lett. 2011;585(1):187–192. doi: 10.1016/j.febslet.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Zheng F, Liao YJ, Cai MY, et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2 [J] Gut. 2011 Jun 14; doi: 10.1136/gut.2011.239145. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Yu F, Yao H, Zhu P, et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells [J] Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 19.Deng YZ, Chen PP, Wang Y, et al. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T- cell factor/Lef signaling [J] J Biol Chem. 2007;282(50):36571–36581. doi: 10.1074/jbc.M704141200. [DOI] [PubMed] [Google Scholar]
- 20.Peng L, Xing X, Li W, et al. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling [J] Mol Cancer. 2009;8:110. doi: 10.1186/1476-4598-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesniak D, Xu Y, Deschenes J, et al. Beta1-integrin circumvents the antiproliferative effects of trastuzumab in human epidermal growth factor receptor-2 – positive breast cancer [J] Cancer Res. 2009;69(22):8620–8628. doi: 10.1158/0008-5472.CAN-09-1591. [DOI] [PubMed] [Google Scholar]
- 22.Samson T, Welch C, Monaghan-Benson E, et al. Endogenous RhoG is rapidly activated after epidermal growth factor stimulation through multiple guanine-nucleotide exchange factors [J] Mol Biol Cell. 2010;21(9):1629–1642. doi: 10.1091/mbc.E09-09-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Wu D, Jin H, et al. Induction of cell retraction by the combined actions of Abl-Crkll and Rho-ROCK1 signaling [J] J Cell Biol. 2008;183(4):711–723. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng R, Cheng L, Shao MY, et al. Roles of lysophosphatidic acid and the Rho-associated kinase pathway in the migration of dental pulp cells [J] Exp Cell Res. 2010;316(6):1019–1027. doi: 10.1016/j.yexcr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Dingemans AM, van den Boogaart V, Vosse BA, et al. Integrin expression profiling identifies integrin alpha5 and beta1 as prognostic factors in early stage non–small cell lung cancer [J] Mol Cancer. 2010;9:152. doi: 10.1186/1476-4598-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prieto-Sanchez RM, Berenjeno IM, Bustelo XR. Involvement of the Rho/Rac family member RhoG in caveolar endocytosis [J] Oncogene. 2006;25(21):2961–2973. doi: 10.1038/sj.onc.1209333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babeto E, Conceicao AL, Valsechi MC, et al. Differentially expressed genes in giant cell tumor of bone [J] Virchows Arch. 2011;458(4):467–476. doi: 10.1007/s00428-011-1047-4. [DOI] [PubMed] [Google Scholar]
- 28.Lv XB, Xie F, Hu K, et al. Damaged DNA-binding protein 1 (DDB1) interacts with Cdh1 and modulates the function of APC/CCdh1 [J] J Biol Chem. 2010;285(24):18234–18240. doi: 10.1074/jbc.M109.094144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions [J] Nat Genet. 2005;37(5):495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 30.Grimson A, Farh KK, Johnston WK, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing [J] Mol Cell. 2007;27(1):91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chien WW, O'Kelly J, Lu DN, et al. Expression of connective tissue growth factor (CTGF/CCN2) in breast cancer cells is associated with increased migration and angiogenesis [J] Int J Oncol. 2011;38(6):1741–1747. doi: 10.3892/ijo.2011.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debily MA, Camarca A, Ciullo M, et al. Expression and molecular characterization of alternative transcripts of the ARHGEF5/TIM Oncogene specific for human breast cancer [J] Hum Mol Genet. 2004;13(3):323–334. doi: 10.1093/hmg/ddh024. [DOI] [PubMed] [Google Scholar]
- 33.Ying H, Biroc SL, Li WW, et al. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models [J] Mol Cancer Ther. 2006;5(9):2158–2164. doi: 10.1158/1535-7163.MCT-05-0440. [DOI] [PubMed] [Google Scholar]
- 34.Fowler A, Thomson D, Giles K, et al. miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion [J] Eur J Cancer. 2011;47(6):953–963. doi: 10.1016/j.ejca.2010.11.026. [DOI] [PubMed] [Google Scholar]