ABSTRACT
An epidemiological study and control trial were conducted to assess taeniid infection in farm dogs in Qinghai Province, China. To improve egg detection by fecal examination, a deworming step with praziquantel was incorporated into the sampling methodology. As a result, a marked increase in the number of egg-positive samples was observed in samples collected at 24 hr after deworming. Then, the fecal examination and barcoding of egg DNA were performed to assess the prevalence of taeniid species in dogs from Xinghai, Haiyan, Gangcha and Chengduo counties. Analysis of 277 dog feces revealed that taeniid cestodes, including Taenia spp. and Echinococcus granulosus, were highly prevalent in Xinghai (34.4%), but eggs were not found in Haiyan where a control trial on canine echinococcosis had been conducted 20 years previously. A control trial involving the administration of 5–10 mg/kg praziquantel to 90 farm dogs at 45-day intervals was conducted in Xinghai. The prevalence of taeniid cestodes in the dogs was reduced to 9.6% and 4.9% after one and two years, respectively, indicating that some dogs were not administered praziquantel properly. A questionnaire survey of farmers in Xinghai and Haiyan revealed that most farmers in Xinghai were not familiar with echinococcosis or the transmission route of the disease, while most farmers in Haiyan had a more thorough understanding of the disease. The findings implied that a program for educating local farmers would be important for efficiently controlling canine taeniid infection in the region.
Keywords: canine, China, control, Echinococcus, taeniid cestodes
The Chinese province of Qinghai on the Qinghai-Tibetan Plateau is one of the most highly endemic areas for Echinococcus in the world [5] with E. granulosus, E. multilocularis and E. shiquicus having a sympatric distribution in the region [13, 21, 27]. Nomadism of high-altitude adaptive animals, such as yak and sheep, is very popular, and most farmers have dogs for protection and for herding. Together, these conditions favor both endemism and the lifecycle of E. granulosus. Dogs are also infected with E. multilocularis by eating rodents in the pasture.
The nomadic lifestyle of Tibetan inhabitants is considered to be one of major risk factors associated with Echinococcus infection [26], and the reported prevalence in Tibetan nomads reached to 6.1% with E. granulosus and 5.1% with E. multilocularis in endemic areas in Qinghai Province [9]. The prevalence in domestic animals was also high, and the reported value reached to over 50% with E. granulosus and 5% with E. multilocularis in dogs [5, 25] and over 50% with E. granulosus both in yaks and sheep [24, 30, 31]. Dogs also serve as the definitive host of Taenia spp. that can infect to domestic animals, such as yaks and sheep, and thus would reduce animal health and meat quality. Taenia spp., such as T. hydatigena and T. multiceps, were commonly found in sheep and yaks at abattoirs in Qinghai Province [11, 12, 20].
Various control trials on taeniid cestodes, especially E. granulosus, incorporating periodic anthelmintic dosing have been conducted on dogs. In some countries, such as Iceland, New Zealand and Tasmania in Australia, government campaigns achieved complete or provisional eradication of E. granulosus [17]. However, in most endemic areas, efficient control has not been achieved or even attempted. In Qinghai Province, a similar trial combined with an education program was conducted in Haiyan County between 1991 and 1994. In that study, the prevalence of Echinococcus spp. in dogs and sheep was reduced from 63.6% to 0% and 87% to 10%, respectively [14]. The local government supported this campaign, and local veterinarians and epidemic prevention officers properly administered individual dogs with praziquantel at monthly intervals. However, after the termination of the trial, the prevalence in sheep increased to 46.1% by 2009 [14]. No follow-up study on changes in Echinococcus prevalence was conducted in dogs.
A variety of methods have been used to diagnose taeniid infection in dogs. Fecal egg examination is a convenient method for diagnoses in live dogs. The reliability of the method is relatively low, particularly for the diagnosis of tapeworm infection because of its discrete excretion of gravid segments. However, the reliability of this method can be increased, if the feces is collected shortly after deworming [19]. Alternatively, coproantigen and coproDNA detection techniques have been developed and employed in various field studies [6, 7, 10, 19, 29], but reports of cross-reactivity between taeniid species and the presence of inhibitory factors in the feces have meant that the results are not always reliable [6, 15, 16, 23].
In this study, we collected dog feces before and after deworming at a pilot site in Qinghai Province and then determined an optimal sampling schedule for egg examination. Using this method, we evaluated the prevalence of taeniid cestodes in dogs at 4 different sites in Qinghai Province. We then selected the most endemic site and conducted a control trial of taeniid infection in dogs with periodic anthelmintic dosing. Finally, in order to identify fundamental problems with the control trial, we conducted a questionnaire survey to assess farmers’ knowledge of echinococcosis and the management of their dogs.
MATERIALS AND METHODS
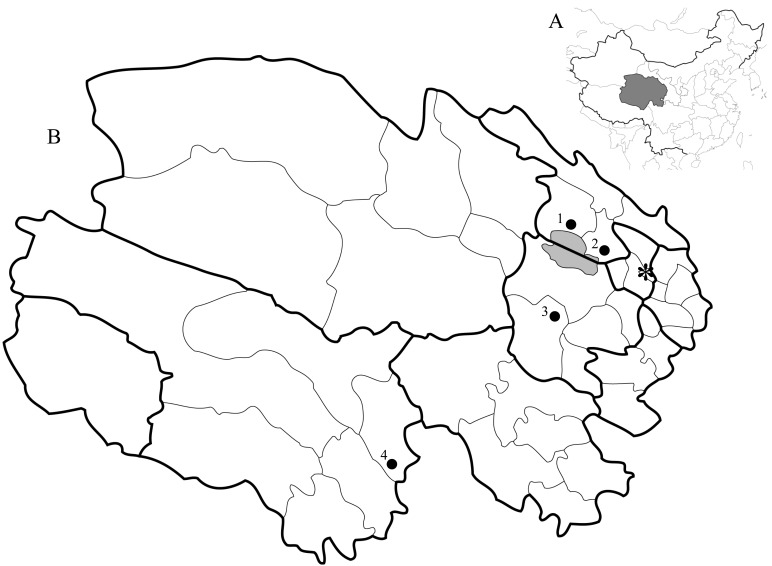
Study sites: This study was conducted in four counties (Gangcha, Haiyan, Xinghai and Chengduo counties) in northeastern and southern Qinghai Province, China (Fig. 1). All of the study sites were inhabited by nomadic Tibetans and other minority ethnic groups grazing yak and sheep on the vast grasslands of the Qinghai-Tibetan Plateau. The altitudes of the study sites ranged from 3,000 to 4,000 m.
Fig. 1.
Study sites. A: Map of China showing Qinghai Province shaded in grey. B: Map of Qinghai Province. Dark area in Map B indicates Qinghai Lake. Bold lines represent district borders, and thin lines represent county borders. 1: Gangcha County, 2: Haiyan County, 3: Xinghai County (Heka Town), 4: Chengduo County. *: Xining (provincial capital).
Collection of dog feces: To collect dog feces, we first asked local farmers to chain their dogs to prevent them from running away to ensure that they would defecate nearby. At Heka Town in Xinghai County, dog feces were collected before administration of 5 to 10 mg/kg praziquantel and then at 24 and 48 hr thereafter to determine the optimal time for detecting eggs from feces by fecal egg examination. Upon finding tapeworms in feces after deworming, they were collected and identified to species by DNA barcoding. Except for Chengduo County where deworming was not performed, dog feces were collected at 24 hr after praziquantel administration in all study sites. All feces samples were frozen at −80°C for at least 10 days to render eggs uninfective.
Fecal egg examination: Fecal egg examination was performed by the centrifugal sucrose flotation technique using 1.0 g of feces. Upon observing taeniid eggs, 2 to 40 eggs were collected from each feces sample under a stereomicroscope, and the eggs were identified to species by DNA barcoding.
Molecular identification of parasite species (DNA barcoding): DNA was extracted from adult cestodes or eggs using a QIAamp DNA Mini Kit (Qiagen, Tokyo, Japan) following the manufacturer’s instructions. As reported previously [8], partial segments of the parasite mitochondrial cytochrome c oxidase subunit 1 (cox1) and NADH dehydrogenase (nad1) gene were amplified by polymerase chain reaction (PCR) using primers 2575 (5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and 3021 (5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′) [3] for cox1 and nad1T-Fw (5′-GGK TAT TCT CAR TTT CGT AAG GG-3′) and nad1T-Rv (5′-ATC AAA TGG AGT ACG ATT AGT YTC AC-3′) [1] for nad1. The amplified products (443 and 507 base pairs for cox1 and nad1, respectively) were examined by agarose gel electrophoresis, and their nucleotide sequences were determined as described previously [8]. The obtained sequences were aligned using CLC Sequence Viewer 6.1 (CLC Bio Japan, Tokyo, Japan), and a Basic Local Alignment Search Tool (BLAST) similarity search was conducted for species and genotype identification.
Deworming of dogs: A control trial to assess taeniid infection in farm dogs was conducted in the town of Heka in Xinghai County where the prevalence of taeniid cestodes in dogs was the highest. A total of 90 dogs were selected for the trial. With support of a local veterinarian, dog owners were requested to administer the required dose of praziquantel (5–10 mg/kg) to each dog at 45-day intervals from July 2010 for two years. Change in the prevalence of infection with taeniid cestodes was then assessed by comparing the prevalence before the beginning of the trial and those at 1 and 2 years after starting the trial. In addition, in order to confirm the efficacy of praziquantel treatment, feces samples were collected and examined again from all dogs 1 month after the 2nd year examination when all dogs were dewormed properly for sampling feces.
Questionnaire survey: Since the disparity in taeniid cestode prevalence in dogs was greatest between the counties of Xinghai and Haiyan, a questionnaire survey was conducted among 30 farmers in these 2 counties to assess their knowledge of echinococcosis and their feeding and contact with their dogs.
Statistical analysis: Difference in the questionnaire responses between the 2 counties was evaluated by Fisher’s exact test implemented in the program R [22]. P<0.05 was considered to be significant.
RESULTS
Fecal egg examination: The results of fecal egg examination performed on feces collected from 43 dogs before praziquantel administration and then at 24 and 48 hr thereafter are shown in Table 1. Taeniid eggs were found in 9 (21%), 18 (43%) and 7 (17%) feces samples, respectively, indicating detection rate was highest in feces collected at 24 hr after deworming. After deworming, 12 dogs excreted tapeworms. Except for one dog, all of these dogs excreted taeniid eggs. DNA barcoding of the excreted eggs and adult cestodes revealed that 18 dogs were infected with T. hydatigena and one dog with T. multiceps. Eggs of other parasite species were not found.
Table 1. Comparison of taeniid egg detection rate by fecal examination in feces samples collected before and after deworming dogs and the number of dogs that excreted cestodes after deworming.
| Taeniid species | No. dogs | No. positive in fecal examination |
No. dogs excreting cestodes |
||
|---|---|---|---|---|---|
| Timing of sampling | |||||
| Before | After 24 hr | After 48 hr | |||
| Taenia hydatigena | 18 | 8 | 17 | 6 | 11 |
| Taenia multiceps | 1 | 1 | 1 | 1 | 1 |
| None | 24 | 0 | 0 | 0 | 0 |
| Total | 43 | 9 (21%) | 18 (43%)* | 7 (17%)* | 12 (29%)* |
*: Because an owner refused to have his dog given anthelmintic, a total number examined was 42.
Prevalence of taeniid cestodes in dogs at four different study sites: The prevalence of taeniid eggs in the feces of dogs from 4 counties examined in 2010 is shown in Table 2. The highest prevalence was found in Xinghai County, and no positive samples were found in Haiyan County. DNA barcoding of egg DNA showed that E. granulosus and T. hydatigena were detected in 3 of the counties and that Xinghai County had the highest prevalence of both parasite species. All nad1 sequences of E. granulosus were identical to that registered in GenBank as G1 genotype (accession No. JF946624). In Gangcha and Xinghai counties, T. multiceps was detected in 1 dog feces sample in each county. In addition to taeniid eggs, Trichuris vulpis eggs were found in 1 dog from Gangcha County and in 2 dogs from Xinghai County.
Table 2. Prevalence of taeniid cestodes determined by fecal egg examination in dogs from four counties in Qinghai Province, China.
| County | No. dogs examined |
No. positive | Taeniid species |
||
|---|---|---|---|---|---|
| Echinococcus granulosus | Taenia hydatigena | Taenia multiceps | |||
| Haiyan*a) | 38 | 0 | NA | NA | NA |
| Gangcha*a) | 77 | 14 | 1 | 12 | 1 |
| Xinghai*a) | 90 | 31 | 12 | 18 | 1 |
| Chengduo*b) | 72 | 9 | 6 | 3 | 0 |
| Total | 277 | 54 | 19 | 33 | 2 |
NA: Not applicable. *a) Fecal egg examination with deworming. *b) Fecal egg examination without deworming.
Control trial of taeniid infection in dogs: Although the trial was started with 90 dogs, the number of animals decreased to 41 over the course of the trial due to death of dogs, transfer of dogs, not having of dogs chained at the time of sampling, disappearance of dogs and owners and owners rejecting further deworming treatments because of changes in dog health after dosing (Table 3). The overall prevalence of taeniid cestodes was reduced from 34.4% at the beginning of the trial to 9.6% and to 4.9% 1 and 2 years after starting the trial, respectively. Changes were also observed in the prevalence of individual parasites. For example, E. granulosus prevalence changed from 13.3% to 3.6% and to 2.4%, T. hydatigena prevalence changed from 20.0% to 2.4% and to 2.4%, and T. multiceps prevalence changed from 1.1% to 3.6% and to 0% 1 and 2 years after starting the trial, respectively. Only 2 dogs were positive for taeniid eggs in consecutive years of the study, but both dogs were infected by different taeniid species in different years; 1 dog was infected with T. hydatigena before anthelmintic treatment, but with E. granulosus at the end of the first year, and the other dog was infected with E. granulosus at the end of the 1st year, but with T. hydatigena at the end of the 2nd year. When dogs were examined for the last time, i.e. 1 month after the 2nd year examination, no eggs were detected in any of the dog feces.
Table 3. Change in taeniid cestode prevalence in dogs over the course of a 2-year anthelmintic control trial in Xinghai County, China.
| Taeniid species | Pre-trial | One year post-trial | Two years post-trial | |||
|---|---|---|---|---|---|---|
| No. positive /No. examined | Prevalence (%) | No. positive /No. examined | Prevalence (%) | No. positive /No. examined | Prevalence (%) | |
| E. granulosus | 12 / 90 | 13.3 | 3 / 83 | 3.6 | 1 / 41 | 2.4 |
| T. hydatigena | 18 / 90 | 20.0 | 2 / 83 | 2.4 | 1 / 41 | 2.4 |
| T. multiceps | 1 / 90 | 1.1 | 3 / 83 | 3.6 | 0 | 0 |
| Total | 31 / 90 | 34.4 | 8 / 83 | 9.6 | 2 / 41 | 4.9 |
Farmers’ awareness of echinococcosis and animal management: The responses to the questionnaire are shown in Table 4 . Although obvious differences were not observed in the feeding behavior of the respective dogs or contact frequency with their dogs, significant difference was observed in the awareness of echinococcosis and the knowledge of transmission risk and lifecycle.
Table 4. Comparison of questionnaire responses from farmers in Haiyan and Xinghai counties.
DISCUSSION
Infection by E. granulosus and T. hydatigena in dogs was detected in 3 of the 4 counties, indicating the species are distributed widely in Qinghai Province. These findings are consistent with reports of cystic echinococcosis in humans and domestic animals elsewhere on the Qinghai-Tibetan Plateau [5, 9, 13, 14, 26, 29,30,31]. Finding T. hydatigena metacestodes at abattoirs seems to be very common, and the prevalence was reported to be 8.2–30.8% in sheep [11, 20]. While, T. multiceps was only found in 2 dogs, and the prevalence of this parasite in Qinghai Province is moderate and has been reported in 1.44 and 3.79% of the sheep and yaks examined, respectively [12]. However, the pathogenicity associated with T. multiceps infection in sheep is very high with 97% of the Tibetan sheep showing clinical signs dying after developing neurologic disorders due to cerebral coenurosis [12]. Measures should therefore be implemented to prevent infection of dogs both by Echinococcus spp. and T. multiceps.
Other species of Echinococcus were not detected in this study. However, previous reports showed that the prevalence of E. multilocularis in humans and dogs was 1.3–3.3 and 2.5–3.1%, respectively, in southern Qinghai Province where Chengduo County is located [25, 29]. Infection with E. shiquicus was not detected either. This parasite is exclusively reported in Qinghai-Tibetan Plateau and maintained by Tibetan sand fox and plateau pika [27]. Dogs are not considered to be a suitable host for this parasite [28]. Recently, however, E. shiquicus DNA was found in the feces of dogs [2]. This suggests the possibility of the parasite infecting to dogs, although the coproDNA found in dog feces may have been derived from a diet.
Mixed infection, i.e. infection with multiple species of taeniid cestodes, was not detected in this study. However, it should be noted that the method used to identify taeniid species in this study could not always detect cases of mixed infection because the DNA examined was obtained from up to 40 eggs.
In Haiyan County, no eggs were found in the 38 dog feces samples examined. Although an anthelmintic dosing campaign in this county was terminated in 1994, some of the local residents were aware of the risk of echinococcosis and continued to dose their dogs. Following a report stating that the prevalence of E. granulosus in yaks and sheep increased after the termination of the campaign [14], the current prevalence in dogs is unlikely to be 0%; nonetheless, it is expected to be low. Our observation was contradicted with the previous observation in Peru where the termination of a control program may have contributed to a marked increase in infection prevalence in intermediate and definitive hosts as well as in humans [17, 18].
The sensitivity with which parasite eggs, especially cestode eggs, can be detected by fecal examination increases shortly after anthelmintic dosing of animals [19]. In this study, taeniid eggs were most frequently detected in dog feces collected 24 hr after dosing. In the 1 dog from which no taeniid eggs were obtained, a tapeworm (T. hydatigena) was excreted after praziquantel dosing. The failure to detect eggs in this animal may have been due to infection with an immature tapeworm, but we did not check to see if the excreted tapeworm contained any mature eggs (gravid segments).
The control trial conducted as part of this study reduced the prevalence of taeniid cestodes in the dogs examined. However, it was appeared that some of the dog owners did not dose their dogs properly because taeniid cestodes including E. granulosus and T. hydatigena were still detected in 9.6% and 4.9% of the dogs at the end of the 1st and 2nd years of the trial. Since the prepatent periods of E. granulosus, T. hydatigena and T. multiceps are about 45–60, 51 and 30 days, respectively [4], praziquantel doses with 45-day intervals can allow only T. multiceps to become patent infection during the trial. In addition, the observation that no taeniid eggs were detected in any of the dog feces samples one month after the final examination at the end of 2nd year when all of the dogs were properly dewormed using praziquantel, indicated that praziquantel was highly effective for treating taeniid cestodes.
The number of dogs used for the control trial was reduced from 90 to 41 at the end of the trial. Although a variety of reasons were responsible for this decrease in the number of animals, we did not anticipate such a marked reduction at the beginning of the trial. Many of the farmers in the study obtained new dogs to replace those that went missing, and it appears that replacing or trading dogs is common among farmers; this practice should be therefore considered, if controls are designed in the future.
The questionnaire survey revealed that an awareness of the risk of echinococcosis infection in humans, as well as knowledge of the transmission route of the parasite, would likely affect the efficacy of any control measures. For example, in Heka Town, where the control trial of this study was conducted and where local farmers did not know much about echinococcosis, deworming of all of the target dogs was not achieved. Conversely, in Haiyan County where a control trial combined with an education program had been conducted previously and where the level of disease awareness among local farmers was high, taeniid eggs were not detected in any of the dog feces samples examined. These findings suggested that education programs should be an integral component of any measures designed to control canine echinococcosis or initiatives directed at reducing the prevalence of Taenia infection in dogs.
Acknowledgments
We are grateful to the staff of the Laboratory of Veterinary Parasitic Diseases, Department of Veterinary Sciences, Faculty of Agriculture, University of Miyazaki and the staff of Academy of Animal and Veterinary Medicine, University of Qinghai for their valuable support. This work was supported by the Strategic Japanese-Chinese Cooperative Program of Japan Science and Technology Agency (JST) and by the Integrated Research Project for Human and Veterinary Medicine in University of Miyazaki funded by the Ministry of Education, Culture, Sports, Science & Technology in Japan.
REFERENCES
- 1.Armua-Fernandez M. T., Nonaka N., Sakurai T., Nakamura S., Gottstein B., Deplazes P., Phiri I. G. K., Katakura K., Oku Y.2011. Development of PCR/dot blot assay for specific detection and differentiation of taeniid cestode eggs in canids. Parasitol. Int. 60: 84–89. doi: 10.1016/j.parint.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Boufana B., Qiu J., Chen X., Budke C. M., Campos-Ponce M., Craig P. S.2013. First report of Echinococcus shiquicus in dogs from eastern Qinghai-Tibet Plateau region, China. Acta Trop. 127: 21–24. doi: 10.1016/j.actatropica.2013.02.019 [DOI] [PubMed] [Google Scholar]
- 3.Bowles J., Blair D., McManus D. P.1995. A molecular phylogeny of the genus Echinococcus. Parasitology 110: 317–328. doi: 10.1017/S0031182000080902 [DOI] [PubMed] [Google Scholar]
- 4.Bowman D. D.2009. Georgis’ parasitology for Veterinarians. 9th ed. Saunders Elsevier, St. Louis. [Google Scholar]
- 5.Craig P. S. and Echinococcosis Working Group in China. 2006. Epidemiology of human alveolar echinococcosis in China. Parasitol. Int. 55: S221–S225. doi: 10.1016/j.parint.2005.11.034 [DOI] [PubMed] [Google Scholar]
- 6.Deplazes P., Eckert J.1996. Diagnosis of the Echinococcus multilocularis infection in final hosts. Appl. Parasitol. 37: 245–252 [PubMed] [Google Scholar]
- 7.Deplazes P., Gottstein B., Eckert J., Jenkins D. J., Ewald D., Jimenez-Palacios S.1992. Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitol. Res. 78: 303–308. doi: 10.1007/BF00937088 [DOI] [PubMed] [Google Scholar]
- 8.Guo Z. H., Kubo M., Kudo M., Nibe K., Horii Y., Nonaka N.2011. Growth and genotypes of Echinococcus granulosus found in cattle imported from Australia and fattened in Japan. Parasitol. Int. 60: 498–502. doi: 10.1016/j.parint.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Han X., Wang H., Qiu J., Ma X., Cai H., Liu P., Ding Q., Dai N., Ito A., Craig P. S.2006. Epidemiological study on Echinococcus multilocularis and E. granulosus in Banma County of Qinghai Province. Chin. J. Zoon. 22: 189–190 (in Chinese). [Google Scholar]
- 10.Huang Y., Yang W., Qiu J., Chen X., Yang Y., Qiu D., Xiao N., Xiao Y., Heath D.2007. A modified coproantigen test used for surveillance of Echinococcus spp. in Tibetan dogs. Vet. Parasitol. 149: 229–238. doi: 10.1016/j.vetpar.2007.08.026 [DOI] [PubMed] [Google Scholar]
- 11.Li C., Dong W., Xie Q., Zhan S., Hong Y.1996. Investigation the prevalence of Echinococcus granulosus and Taenia hydatigena infection of yaks and sheep in Delingha city. Chin. J. Anim. Hlth. Inspec. 13: 25–26 (in Chinese). [Google Scholar]
- 12.Li L. F.2011. Investigation of Taenia multiceps infection in yaks of Qinghai Province. Chin. Qinghai J. Anim. Vet. Sci 41: 25–26 (in Chinese). [Google Scholar]
- 13.Li T. Y., Qiu J. M., Yang W., Craig P. S., Chen X. W., Xiao N.2005. Echinococcosis in Tibetan populations, western Sichuan province, China. Emerg. Infect. Dis 11: 1866–1873. doi: 10.3201/eid1112.050079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y. F., Li D. S.2010. An epidemiological study on echinococcosis in Haiyan County. China Academ. J. Electronic. Pub 27: 38–39 (in Chinese). [Google Scholar]
- 15.Malgor R., Nonaka N., Basmadjian I., Sakai H., Carambula B., Oku Y., Carmona C., Kamiya M.1997. Coproantigen detection in dogs experimentally and naturally infected with Echinococcus granulosus by a monoclonal antibody-based enzyme-linked immunosorbent assay. Int. J. Parasitol. 27: 1605–1612. doi: 10.1016/S0020-7519(97)00127-6 [DOI] [PubMed] [Google Scholar]
- 16.Monteiro L., Bonnemaison D., Vekris A., Petry K. G., Bonnet J., Vidal R., Cabrita J., Mégraud F.1997. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J. Clin. Microbiol. 35: 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moro P., Schantz P. M.2009. Echinococcosis: a review. Int. J. Infect. Dis. 13: 125–133. doi: 10.1016/j.ijid.2008.03.037 [DOI] [PubMed] [Google Scholar]
- 18.Moro P. L., McDonald J., Gilman R. H., Silva B., Verastegui M., Malqui V., Lescano G., Falcon N., Montes G., Bazalar H.1997. Epidemiology of Echinococcus granulosus infection in the central Peruvian Andes. Bull. World Health Organ. 75: 553–561 [PMC free article] [PubMed] [Google Scholar]
- 19.Nonaka N., Kamiya M., Kobayashi F., Ganzorig S., Ando S., Yagi K., Iwaki T., Inoue T., Oku Y.2009. Echinococcus multilocularis infection in pet dogs in Japan. Vector-borne Zoon. Dis. 9: 201–205 [DOI] [PubMed] [Google Scholar]
- 20.Qi Y., Xu M., Yan Y.1992. Investigation on the prevalence of Taenia hydatigena infection of sheep in Tianjun County. Chin. Qinghai J. Anim. Vet. Sci. 22: 19 (in Chinese). [Google Scholar]
- 21.Qiu J., Chen X., Ren M., Lou C., Liu D., Liu X., He D.1995. Epidemiological study on alveolar hydatid disease in Qinghai-Xizang Plateau. J. Pract. Parasit. Dis. 3: 106–109 (in Chinese with English summary). [Google Scholar]
- 22.R Development Core Team2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051-07–0, URL http://www.R-project.org/
- 23.Raoul F., Deplazes P., Nonaka N., Piarroux R., Vuitton D. A., Giraudoux P.2001. Assessment of the epidemiological status of Echinococcus mulitlocularis in foxes in France using ELISA coprotests on fox faeces collected in the field. Int. J. Parasitol. 31: 1579–1588. doi: 10.1016/S0020-7519(01)00280-6 [DOI] [PubMed] [Google Scholar]
- 24.Tan S., Chen G.2005. Study on the prevalence of echinococcosis in yaks and sheep at Gangcha, China. Chin. Qinghai J. Anim. Vet. Sci. 35: 21 (in Chinese). [Google Scholar]
- 25.Wang H., Zhang J. X., Schantz P. M., Ito A., Craig P. S., Wu X. H., Han X. M.2006. Epidemiologic survey and analysis on echinococcosis in humans and animals from 1995 to 2005 in Qinghai Province. Chin. J. Zoon 22: 1129–1134 (in Chinese with English summary). [Google Scholar]
- 26.Wang Z., Wang X., Liu X.2008. Echinococcosis in China, a review of the epidemiology of Echinococcus spp. EcoHealth 5: 115–126. doi: 10.1007/s10393-008-0174-0 [DOI] [PubMed] [Google Scholar]
- 27.Xiao N., Qiu J., Nakao M., Li T., Yang W., Chen X., Schantz P. M., Craig P. S., Ito A.2005. Echinococcus shiquicus n. sp., a taeniid cestode from Tibetan fox and plateau pika in China. Int. J. Parasitol. 35: 693–701. doi: 10.1016/j.ijpara.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 28.Xiao N., Qiu J., Nakao M., Li T., Yang W., Chen X., Schantz P. M., Craig P. S., Ito A.2006. Echinococcus shiquicus, a new species from the Qinghai-Tibet plateau region of China: Discovery and epidemiological implications. Parasitol. Int. 55: (Suppl.): S233–S236. doi: 10.1016/j.parint.2005.11.035 [DOI] [PubMed] [Google Scholar]
- 29.Yu S. H., Wang H., Wu X. H., Ma X., Liu P. Y., Liu Y. F., Zhao Y. M., Morishima Y., Kawanaka M.2008. Cystic and alveolar echinococcosis: an epidemiological survey in a Tibetan population in Southeast Qinghai, China. Jpn. J. Infect. Dis. 61: 242–246 [PubMed] [Google Scholar]
- 30.Zhang J. X., Wang H.2007. Epidemiological Survey on Echinococcus Infection in Animals in Qinghai Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 25: 350–352 (in Chinese with English summary). [PubMed] [Google Scholar]
- 31.Zhang X. Q., Wanma D. Z.2007. Study on the prevalence of echinococcosis in yaks at Tianjun County, China. Chin. J. Anim. Health Inspection 24: 36 (in Chinese). [Google Scholar]