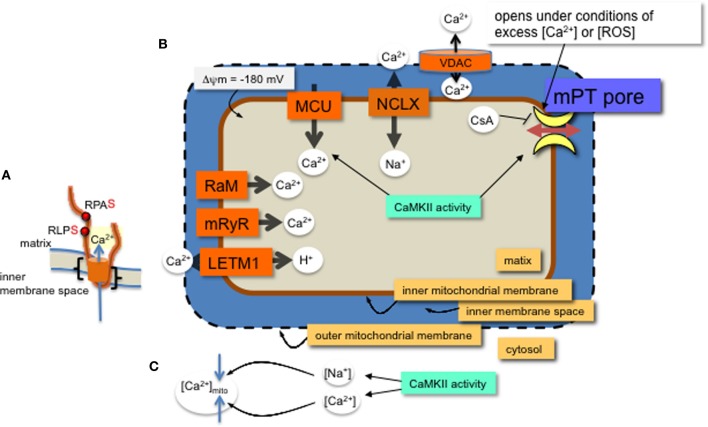
Figure 1.
MCU channel in the inner mitochondrial membrane. (A) A single monomer of MCU is shown (orange) with two phospho-serine residues (red dots, with consensus amino acids adjacent) on the N-terminal region in the matrix. The two transmembrane domains are indicated (black brackets). Layers of regulation include accessory proteins and a protein similar to MCU, MCUb (not shown). (B) Mitochondrial Ca2+ channels and exchangers on the inner membrane. Ca2+ predominantly enters the matrix through the MCU channel and efflux is via the Na+-Ca2+ exchanger (NCLX). Other channels and an exchanger that were found to regulate Ca2+ across the inner membrane are the rapid mode of uptake (RaM), the ryanodine receptor (mRyR) and the Ca2+-H+ exchanger (LETM1). Voltage dependent anion channels (VDAC) allow ions and metabolites across the outer membrane. The proton (H+) gradient, a major component of the membrane potential (ΔΨ), is generated from the electron transport chain and drives the flow of H+ through ATP synthase in a reaction coupled to the generation of ATP from ADP and inorganic phosphate. The membrane potential produces a driving force for matrix Ca2+ accumulation. Excess Ca2+ or ROS will open the permeability transition pore, which can be inhibited with CsA or CaMKIIN. Mitochondrial CaMKII activity regulates 1. Ca2+ entry through the MCU and 2. transition pore opening. (C) CaMKII activity in the cytosol can increase both Ca2+ and Na+ ion levels in the cytosol with opposite effects on mitochondrial matrix Ca2+ accumulation.