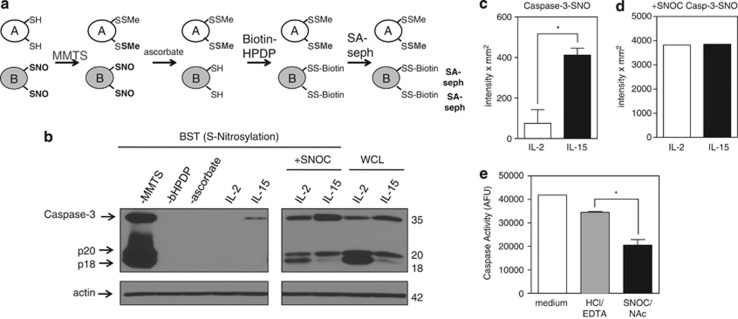
Figure 6.
IL-15 inactivates caspase-3 by S-nitrosylation. (a) Model of the biotin switch technique (BST) that converts S-nitrosylated thiols to biotinylated thiols. In step 1, free thiols are blocked with a thiol-specific methylthiolating agent, methylmethanethiosulfonate (MMTS). In step 2, ascorbate specifically reduces nitrosothiol bonds to thiols. In step 3, N-[6-(biotinamido)hexyl]-3′-(2′- pyridyldithio)propionamide (bHPDP; biotin-HPDP), a sulfydryl-specific biotin linker, biotinylates secondarily formed free thiols. Biotinylated proteins are then precipitated using streptavidin-sepharose beads, separated by gel electrophoresis, and examined by immunoblot for protein of interest. (b) S-nitrosylation of caspase-3 (caspase-3-SNO) was detected by BST in T cells expanded in IL-2 or IL-15 for 5 days. Equal loading of protein was verified by immunoblotting for actin. Caspase-3-SNO was detected by BST. The −MMTS, −bHPDP, and −ascorbate represent controls without thiol blocking agent, biotin linker, or reducing agent, respectively. (c and d) Densitometry of IL-2- and IL-15-cultured T cells immunoblotted for total caspase-3-SNO. Values are mean±S.E.M. Statistical analysis was based on two independent experiments combined using Student's t-test (*P<0.05). (e) Measurement of caspase activity by DEVD-rhodamine assay of IL-2-cultured T cells treated with medium, vehicle control HCl/EDTA, or SNOC/NAc. Results are based on two combined independent experiments. Values are mean±S.E.M. Data were analyzed by Student's t-test (*P<0.05)