Table 11.
Nok vs. TPGS-750-M in amination reactions
|
| ||||||
|---|---|---|---|---|---|---|
| Entry | Aryl halide | Partner | Product | TPGS-750-Ma yield(%) | Noka yield(%) | Time (h) |
| 1b |

|
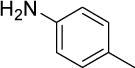
|

|
95 | 91 | 21 |
| 2b |

|

|

|
75 | 75 | 10 |
| 3c,d |

|

|

|
85 | 85 | 24 |
| 4c,e |

|

|

|
96 | 93 | 24 |
Isolated yields.
Reaction conditions: aryl bromide (1.00 mmol, 1 equiv), toluidine (1.20 mmol, 1.2 equiv), catalyst [(π-allyl)PdCl]2 (0.5 mol %), cBRIDP (2 mol %), K-O-t-Bu (1.5 equiv) and 2 wt % of surfactant/H2O; at rt.
Na-O-t-Bu (1.5 equiv); reaction was run at 50 °C, 24 h using [(π-allyl)PdCl]2 (0.5 mol %), cBRIDP (2 mol %), K-O-t-Bu (1.5 equiv) and 2 wt % of surfactant/H2O.
Reaction conditions: aryl bromide (1.00 mmol, 1 equiv), t-butyl carbamate (1.50 mmol, 1.5 equiv).
Reaction conditions: aryl bromide (0.50 mmol, 1 equiv), ethyl carbamate (0.60 mmol, 1.2 equiv).
