Table 3.
Cross- and Ring-Closing Metathesis: Nok vs. TPGS-750-M
|
| ||||||
|---|---|---|---|---|---|---|
| Entry | Alkene | Partner | Product | TPGS-750-M yield(%)a | Nok yield(%)a | Time (h) |
| 1b |

|

|

|
72 | 74 | 12 |
| 2c |

|

|

|
89 | 86 | 12 |
| 3c |

|

|
|
81 | 84 | 24 |
| 4d |

|

|
81 | 88 | 6 | |
| 5d |
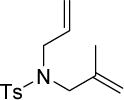
|

|
98 | 96 | 18 | |
Isolated yield of chromatographically pure materials.
Conditions: alkene (0.5 mmol, 1 equiv), MVK (1.5 mmol, 3 equiv).
Reaction: alkene (0.5 mmol, 1 equiv), acrylate (1.0 mmol, 2 equiv).
Reaction: diene (0.25 mmol).
