Abstract
Numerous studies suggest that anxious individuals are more hypervigilant to threat in their environment than nonanxious individuals. In the present event-related potential (ERP) study, we sought to investigate the extent to which afferent cortical processes, as indexed by the earliest visual component C1, are biased in observers high in fear of specific objects. In a visual search paradigm, ERPs were measured while spider-fearful participants and controls searched for discrepant objects (e.g. spiders, butterflies, flowers) in visual arrays. Results showed enhanced C1 amplitudes in response to spatially directed target stimuli in spider-fearful participants only. Furthermore, enhanced C1 amplitudes were observed in response to all discrepant targets and distractors in spider-fearful compared with non-anxious participants, irrespective of fearful and non-fearful target contents. This pattern of results is in line with theoretical notions of heightened sensory sensitivity (hypervigilance) to external stimuli in high-fearful individuals. Specifically, the findings suggest that fear facilitates afferent cortical processing in the human visual cortex in a non-specific and temporally sustained fashion, when observers search for potential threat cues.
Keywords: emotion, hypervigilance, spatial attention, C1, event-related potentials (ERPs)
INTRODUCTION
Voluntary attention to a specific location in the environment results in faster detection and enhanced discrimination for stimuli presented at that location than stimuli at unattended locations. This well-known effect of spatial attention has been demonstrated in striate and extrastriate visual cortical areas (V1–V4; Kastner et al., 1999; Martinez et al., 1999), resulting in increased electrophysiological and hemodynamic activity for attended compared with unattended locations. Recently, Kelly et al. (2008) showed that spatial attention may modulate the initial passage of visual input to primary visual cortex (V1). Using event-related potentials (ERPs), the authors observed enhanced amplitudes of the C1 component in response to spatially cued patterns ∼50 ms after presenting the visual stimulus. The C1 component has been described for many decades as the earliest cortical component in the visual evoked potential (Regan, 1989). Extensive evidence points to the striate cortex as the neural generator of the C1 (Jeffreys and Axford, 1972, Clark et al., 1995), consistent with its peak latencies ∼50–100 ms. The C1 topography evinces pronounced retinotopy, with a voltage reversal over posterior scalp sites reliably related to the location of the stimulus in the upper versus lower visual field. Although best established with systematic manipulation of visual field location, pattern onset at foveal and symmetric locations has been shown to elicit C1 in a robust fashion, given sufficient signal-to-noise ratio (Regan, 1989). Under these conditions, the C1 tends to be negative and widely distributed in topography (Baseler and Sutter, 1997).
Besides spatial attention and perceptual learning (Bao et al., 2010), the C1 has also been described as sensitive to contextual features such as fearful faces and fear-conditioned stimuli (Pourtois et al., 2004, Stolarova et al., 2006), indicating superior evaluation of biologically relevant stimuli at the earliest stage of cortical processing. If biological significance is capable of biasing sensory neurons, then observers high in specific object fear should show sensory facilitation when expecting to confront the feared object. Presently, however, it is unclear how sensory processing varies with inter-individual fear status.
A substantial amount of studies have found that anxious individuals are more vigilant to environmental threat than non-anxious individuals (see for review Bar-Haim et al., 2007; Cisler and Koster, 2010; Yiend, 2010; Miskovic and Schmidt, 2012). For instance, participants reporting fear of spiders (snakes) detect spider (snake) stimuli more rapidly than non-fearful participants, in visual search paradigms (Öhman et al., 2001). Participants high in specific fear also display enhanced N2pc amplitudes in the ERP, indicating enhanced attention capture by these stimuli (Weymar et al., 2013). In the same vein, high-trait anxious individuals detect angry faces faster than low-trait anxious individuals (Byrne and Eysenck, 1995), a difference not observed for happy faces. Similar results were found for socially anxious individuals who show a preferential processing of angry facial expression relative to happy expressions and also shorter responses for angry relative to disgust expressions (Gilboa-Schechtman et al., 1999), pointing to a greater attention bias in anxious individuals. In addition to rapid initial attention capture by threat-relevant stimuli, other studies have shown that high anxious individuals have difficulties disengaging attention from fear-relevant stimuli once they are detected (e.g. Fox et al., 2002; Gerdes et al., 2008). Furthermore, others have proposed that anxious individuals tend to avoid the threat stimulus immediately after detection (Mogg and Bradley, 1998; Pflugshaupt et al., 2005).
Unspecific hypervigilance or heightened alertness even prior to detecting a threat stimulus has previously been described as characteristic of anxious individuals (Beck et al., 1985; Eysenck, 1992). In this perspective, anxious individuals are constantly looking out for signs of threat or harm in their environment and selectively attend to stimuli signaling possible danger. Specifically, Eysenck proposes a broadening of attention (general hypervigilance) during excessive environmental scanning for threat cues followed by a narrowing of attention when a stimulus is being processed (enhanced selective attention). General sensory hypervigilance has also been framed in the defense cascade model based on work in the animal model of defensive behavior (Fanselow, 1994; Lang et al., 1997). According to this model, defensive behavior is characterized by a dynamic sequence of stages: In an initial pre-encounter stage, the organism is subject to a certain risk (e.g. being in an environment where a threat has been previously encountered), but no immediate danger is present (e.g. the predator has not been detected yet). This stage is characterized by physiological changes that are consistent with threat-unspecific hypervigilance to all stimuli in the environment. As soon as the threat is detected, the stages of post-encounter and, with increasing proximity of the threat, finally of active defensive behavior (fight or flight) are initiated, with sensory perception and motor processes increasingly directed toward adaptive action (Keil et al., 2010). Recent ERP studies with specific phobia individuals indicate that spider fearful and (socially) anxious show enhanced P1 amplitudes to all visual stimuli, irrespective of content, than control individuals (Kolassa et al., 2006, 2007; Michalowski et al., 2009; Mühlberger et al., 2009; Wieser et al., 2010), indicating that such a state of sensory vigilance might be evident in humans as well in contexts they fear.
The present study examined the extent to which specific fear modulates the sensitivity of early afferent electrocortical activity in V1 in participants with small animal fear. To address this issue, we measured ERPs in spider-fearful and non-fearful participants while they searched for discrepant fear-specific and -unspecific objects in visual arrays.
MATERIALS AND METHODS
Participants
664 university students were screened with a German version of the 31-item Spider Phobia Questionnaire (SPQ; Hamm, 2006). Total SPQ score can range from 0 to 31, with higher scores indicating greater fear of spiders. Scales left incomplete or mismarked were excluded from analysis. The final sample consisted of 541 students. Twenty-five spider-fearful individuals (22 female, 3 male; mean age: 21.1 years; 2 left-handed) and 25 non-anxious control participants (19 female, 6 male; mean age: 24.8 years; 2 left-handed) were selected based on their scoring points. Subjects reporting elevated spider fear were included in the spider-fearful group (mean: 19.64, range: 17–28; s.d.: 3.40) if scores were ≥17 in the SPQ (88th percentile of the distribution). Twenty-five participants reporting lower levels of spider fear (mean: 3.20, range: 0–7; s.d.: 1.80) were in the non-anxious control group with scores ≤7 in the SPQ (56th percentile of the distribution). The distribution of the mean scores of the student sample was similar to normative data of the SPQ (Klorman et al., 1974). Mean scores of the spider-fearful group were slightly lower compared with clinical samples diagnosed with specific phobia animal type (e.g. Mean = 23.76, Fredrikson, 1983; Mean = 23.2, Muris and Merckelbach, 1996). As expected, spider-fearful participants scored significantly higher on the SPQ than the controls [F(1,48) = 455.83, P < 0.001, ηp2 = 0.91]. All had normal or corrected-to-normal visual acuity. All participants gave their informed consent for the protocol approved by the Review Board of the University of Greifswald and received either course credit or financial compensation for participation. The same sample of participants was included in analyses performed in another study (Weymar et al., 2013). Two subjects (one spider-fearful subject) were excluded owing to poor electroencephalogram (EEG) quality.
Stimulus materials and procedure
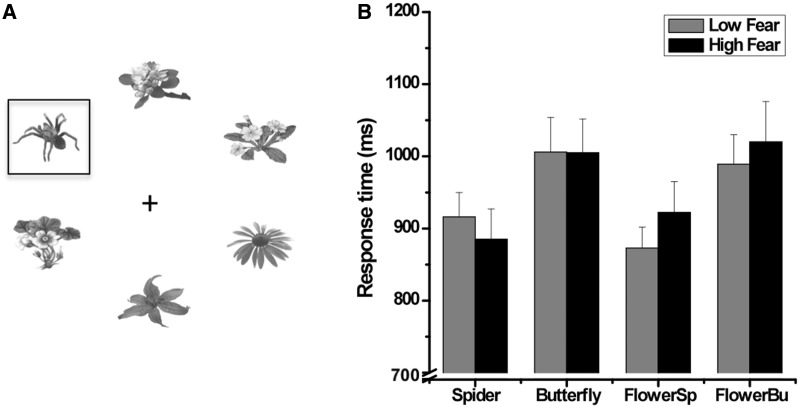
Participants were seated in a dimly lit sound-attenuated cabin, viewing a 20” computer monitor (1024 × 768, 60 Hz) located 1.5 m in front of the viewer. Each search display contained six objects arranged in a circle around a central fixation cross (0.23° width × 0.23° height). The stimuli consisted of spiders, butterflies and flowers (see Gerdes et al., 2008, 2009), which were presented in gray scale and all matched for luminance and contrast. Individual stimuli were 2.10° width × 1.72° height. The distance from the fixation cross to the center of each of the six stimuli was 2.67°. Search displays either contained only distractors (spiders vs butterflies vs flowers) or, among the distractors, there was one discrepant target stimulus from a different category. Overall, seven different arrays (each n = 90) were presented: three arrays with spider, butterfly or flower distractors only and four arrays with a spider or butterfly target among flower objects and a flower target among spider or butterfly objects. The targets occurred 15 times at any of the six positions in the matrix. Target positions were randomized over trials. An example of the stimulus array is given in Figure 1A.
Fig. 1.
(A) Sample of a search array used in the present study. The square marks the target (spider) among various distractors (flowers). (B) Response times for all target conditions [spider targets, butterfly targets, flower targets among spider distractors (FlowerSp) and flower targets among butterfly distractors (FlowerBu)] as a function of group (high fearful vs low fearful). Error bars represent standard error of the means (SEM).
The participants were instructed to detect as quickly and accurately as possible targets from a discrepant category in the arrays, by pressing either a ‘yes’ or ‘no’ button with the left or the right finger (counterbalanced across participants). There were four practice trials with displays containing a target or not.
A trial was initiated by the appearance of a 500 ms fixation cross preceding each stimulus array to ensure that the participants kept their gaze focused on the central fixation location throughout the experiment. The arrays were presented in random order for each participant with the constraint that no array with a target (either spider, butterfly or flower) or no target (spider, butterfly or flower) was presented on more than four consecutive trials. A trial was terminated by the participants’ response, followed by a variable inter-trial interval (ITI) of 1500, 2000 or 2500 ms. Before starting the task, subjects were instructed to avoid eye blinks and excessive body movements during ERP measurement.
Apparatus and data analysis
EEG signals were recorded continuously from 256 electrodes using an Electrical Geodesics (EGI) HydroCel high-density EEG system with NetStation software on a Macintosh computer. The EEG recording was digitized at a rate of 250 Hz, using the vertex sensor (Cz) as recording reference. Scalp impedance for each sensor was kept below 30 kΩ, as recommended by the manufacturer guidelines. All channels were bandpass filtered online from 0.1 to 100 Hz. Stimulus-synchronized epochs were extracted from 100 ms before to 800 ms after picture onset and low-pass filtered (Butterworth filter) at 40 Hz and then submitted to the procedure proposed by Junghöfer et al. (2000), as implemented in the EMEGS software provided by Peyk et al. (2011). This procedure uses statistical parameters of the data to exclude channels and trials that are contaminated with artifacts. Recording artifacts are first detected using the recording reference (i.e. Cz), and then global artifacts are detected using the average reference, which is used for all analyses. In the present study, electrode site Cz was used as the recording reference, and the average reference was calculated off-line, after artifact rejection, and used for all subsequent analyses. Subsequently, distinct sensors from particular trials were removed based on the distribution of their amplitude, standard deviation and gradient. Data at eliminated electrodes were replaced with a statistically weighted spherical spline interpolation from the full channel set (Junghöfer et al., 2000).
For behavioral data, accuracy rates (AR) and response times (RT) of accurate responses were analyzed. Overall, participants responded correctly on 96% of the trials (hits and correct rejections). Response times more than 3 s.d.s above each participant’s mean (see Holmes et al., 2009; Weymar et al., 2011) were excluded to eliminate the influence of outliers (1.8% of data). Behavioral data were submitted to repeated measures ANOVAs using the factors target content (spider vs butterfly vs flower among spiders vs flower among butterflies) and group (spider fearful vs controls). Extensive analyses of the behavioral data are reported elsewhere (Weymar et al., 2013).
ERPs were based on valid trials only (based on hit rate, correct rejection rate and outlier analyses) and were computed for each sensor and participant. Overall, ∼18% of the trials were rejected because of artifacts and outliers in response times. These rejected trials were equally distributed across all categories. In consideration of previous research (Pourtois et al., 2004; Stolarova et al., 2006; Kelly et al., 2008) showing an occipito-parietal distribution of the C1 component and based on visual inspection of the waveforms, mean ERP amplitudes were analyzed over posterior electrodes: The C1 was scored on the basis of maximal ERP amplitudes at the EGI sensors 75, 76, 77, 84, 85, 86, 87, 95, 96, 97, 98, 99, 105, 106, 107, 108, 109, 114, 115, 116, 117 (left), and 139, 140, 141, 150, 151, 152, 153, 159, 160, 161, 162, 163, 168, 169, 170, 171, 172, 177, 178, 179, 180 (right), see Figure 2. The timing of the C1 has been established in a plethora of classical studies in visual neuroscience (Spekreijse et al., 1973) and electrophysiology (Clark et al., 1995). We used filter settings similar to Clark et al. (1995), leading to similar timing of the early visual evoked potential (VEP) response: The C1, which tends to peak around 50–70 ms and is often preceded by a slope that starts to differ from baseline in the time range before 50 ms, often around 20–40 ms (Parker et al., 1982, Figure 4; Clark et al., 1995, Figure 4; Rauss et al. 2009, Figure 3, left). This very early segment may contain carry-over from preparatory or motor processes overlapping and interacting with sensory processing (Vogel and Luck, 2000). Because we were interested in hypervigilance and sustained sensory facilitation, we included this very early interval in our analyses. To test for modulation of the C1 by spatial attention, as well as for content and group effects, a repeated measures ANOVA was conducted including target content (spider vs butterfly vs flower among spiders vs flower among butterflies), target location (left vs right relative to fixation) and electrode site (left vs right cluster) as within factors and group (high vs low spider fear) as between factor. Targets located in the upper and lower left position were merged to one single left location condition (n = 30 for each picture), whereas the upper right and lower right target stimuli were averaged for the right location condition (n = 30 for each picture), see Figure 1B. Targets presented at the top or bottom mid screen positions did not enter the analyses.
Fig. 2.
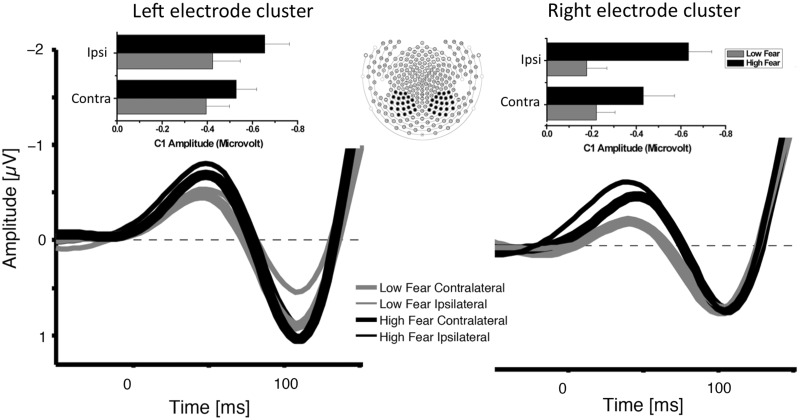
Hypervigilance in spider-fearful subjects. Grand average ERPs elicited in the 150 ms interval after target onset in response to all targets objects contralateral (thick lines) and ipsilateral (thin lines) to the visual hemifield where the target was presented for spider-fearful (black lines) and control (gray lines) participants. ERPs are averaged across electrodes within posterior sensor clusters (middle section). Insets illustrate the mean ERP amplitude (24–40 ms) for targets presented ipsilateral and contralateral to the visual field at the left and right electrode cluster for both groups.
Fig. 4.
Magnitude of the C1 amplitude in response to spider targets, butterfly targets, flower targets among spider distractors (FlowerSp) and flower targets among butterfly distractors (FlowerBu) in the 40–60 ms time window for control (gray bars) and spider-fearful participants (black bars). Error bars indicate SEM.
Fig. 3.
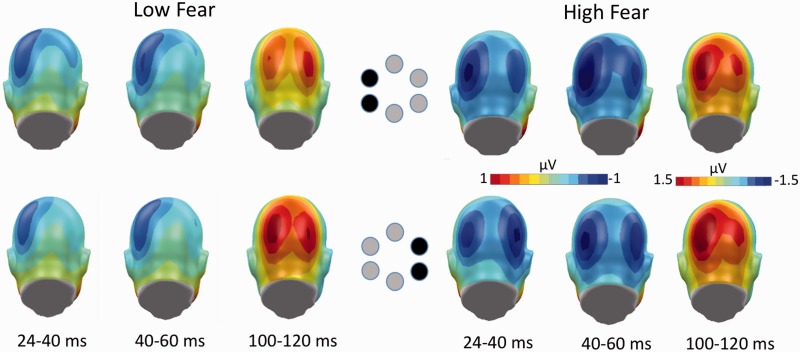
Illustration of the topographical voltage maps (back views) for all target objects, showing the distribution of C1 negativity in the time interval from 24 to 40 ms and from 40 to 60 ms for control (left) and spider-fearful subjects (right). In addition, P100 topographical maps are shown for both experimental groups for the 100–120 ms time window. Upper panel represents the topography for targets presented in the left visual field, whereas the lower panel represents the neural activity in response to targets presented in the right visual field.
To control for unspecific group differences in ERP amplitude owing to inter-individual differences in signal-to-noise or head geometry/anatomy, we included the P1 amplitude in our analyses. The rationale for this was that any group difference that favors the ERP signal would do so for the C1 and the P1, whereas specific effects should affect one component but not the other. Effects of attention, content and group on the P100 component were tested for the 100–120 ms interval, where the P100 amplitude was maximal. As for the C1, ANOVAs with the factors target content, target location, electrode site and group were carried out for the P100 component over posterior electrodes (see above).
Subsequent analysis of the N100 and N2pc component (see Supplementary Material) showing fear-specific effects in the spider-fearful group are reported elsewhere (Weymar et al., 2013).
For effects involving repeated measures, the Greenhouse–Geisser procedure was used to correct for violations of sphericity.
RESULTS
Behavioral data
Accuracy rates and response times
Accuracy differed as a function of target content, F(3,138) = 11.79, P < 0.001, ηp2 = 0.20. Spider targets were detected better (97%) compared with butterflies [96%, F(1,46) = 15.17, P < 0.001, ηp2 = 0.25] and both flower targets [flower targets among butterflies, 94%, F(1,46) = 34.36, P < 0.001, ηp2 = 0.43, and flower targets among spiders, 96%, F(1,46) = 6.42, P < 0.05, ηp2 = 00.12]. Accuracy did not differ between spider-fearful participants and controls [group: F(1,46) = 2.33, P = 0.13, ηp2 = 0.05]. Owing to the high accuracy rate, data of all target conditions were angular transformed using the arc sine square root transformation. Using this normalization, accuracy was still better for spider targets than for butterfly targets [F(1,46) = 7.74, P < 0.001, ηp2 = 0.14], flower targets among butterflies [F(1,46) = 367.36, P < 0.001, ηp2 = 0.44] and flower targets among spiders [F(1,46) = 3.79, P = 0.06, ηp2 = 0.08]. Accuracy did not differ between both experimental groups [group: F(1,46) = 2.03, P = 0.16, ηp2 = 0.04].
The response times are displayed in Figure 1. A main effect of object content was observed [F(3,138) = 29.41, P < 0.001, ηp2 = 0.39]. Follow-up tests showed that response times for spider targets and flower targets surrounded by spider distractors were faster than for butterfly targets [F(1,46) = 35.84, P < 0.001, ηp2 = 0.44, and F(1,46) = 46.10, P < 0.001, ηp2 = 0.50] and flower targets among butterflies [F(1,46) = 30.04, P < 0.001, ηp2 = 0.40, and F(1,46) = 56.86, P < 0.001, ηp2 = 0.55]. Overall, response times did not differ between both groups [F(1,46) < 1, P = 0.83, ηp2 = 0.00]. Response times were also analyzed using quartiles of the distributions across conditions and group. Again, shorter response times were observed for trials containing spiders (either as target or background) in both groups, indicating that the pattern of results (Figure 1) did not differ from faster to slower response times.
ERP data
C1
Base and early slope of the C1
Figure 2 shows the grand average ERPs obtained over parieto-occipital sensor clusters contralateral to the target location and ipsilateral to the target location. As can be seen, a C1 component was elicited in response to the target stimuli, starting at ∼24 ms after stimulus onset over posterior scalp sites. Overall analysis revealed a trend for an interaction between electrode site and target position [F(1,46) = 2.95, P = 0.09, ηp2 = 0.06]. More importantly, significant group effects were observed, showing that spider-fearful participants showed overall a significantly larger C1 amplitude than the control group [F(1,46) = 6.25, P = 0.01, ηp2 = 0.12], irrespective of target content, F(3,138) < 1, P = 0.52, ηp2 = 0.00, see Figure 2. Moreover, spatial position effects were observed for the C1 component in spider-fearful participants as indicated by a significant interaction between target location, electrode site and group, [F(1,46) = 3.55, P = 0.06, ηp2 = 0.07], see Figures 2 and 3. ERP amplitudes were more negative at electrode sites ipsilateral to the position of the target than at contralateral electrodes, target location × electrode site: F(1,23) = 6.18, P = 0.02, ηp2 = 0.21, in fearful individuals. In contrast, control individuals showed no electrode × target interaction, F(1,23) < 1, P = 0.91, ηp2 = 0.00, but overall larger negative ERP amplitudes over the left compared with right electrodes, electrode site: F(1,23) = 3.77, P = 0.06, ηp2 = 0.15.
Peak of the C1
Figure 4 shows the mean ERP amplitude changes for the C1 component as a function of target content and group. In the later time window (40–60 ms), the C1 amplitude continued to be significantly enhanced for the high relative to the low spider-fearful group, F(1,46) = 8.01, P < 0.001, ηp2 = 0.15. C1 modulation was not different for the four object targets [content × group, F(3,138) < 1, P = 0.97, ηp2 = 0.00]. No spatial position effects were significant for the later time window [target × location: F(1,46) = 2.70, P = 0.11, ηp2 = 0.06; target × location × group: F(1,46) < 1, P = 0.33, ηp2 = 0.02].
C1 peak amplitudes in response to the non-target conditions (spiders vs butterflies vs flowers) were also analyzed. Significant group effects were also visible between fearful and non-fearful participants during processing of non-target matrices [F(1,46) = 5.05, P < 0.05, ηp2 = 0.10]. A repeated measures ANOVA with the factor target (target vs non-target) and group revealed a significant group effect, F(1,46) = 7.26, P = 0.01, ηp2 = 0.14, but no significant target effects or interactions [target: F(1,46) < 1, P = 54, ηp2 = 0.01; target × group: F(1,46) < 1, P = 0.80, ηp2 = 0.00], showing that the enhanced sensory processing in V1 neurons in fearful participants is not specifically related to targets but instead is evident for all presented visual arrays.
Correlations between behavioral measures and C1 amplitude
The detection accuracy was negatively correlated with the amplitude of the C1 (Slope and Peak) in response to all target conditions in spider-fearful individuals (Slope: r = −0.48, one-tailed P < 0.01; Peak: r = −0.38, one-tailed P < 0.05) but not in non-fearful individuals (Slope: r = −0.19, one-tailed P = 0.19; Peak: r = −0.01, one-tailed P = 0.48), indicating that higher accuracy was associated with larger C1 amplitude in spider-fearful participants.
P100
Peak of the P1
The P100 component was peaking in the 100–120 ms time window over posterior scalp sites (see Figures 2 and 3). A main effect of target location indicated larger P1 amplitudes in response to targets presented in the right visual field compared with targets presented in the left visual field, F(1,46) = 4.28, P < 0.05, ηp2 = 0.09. Critically, as opposed to the C1 component, P100 amplitudes did not differ between spider fearful and non-fearful participants, F(1,46) < 1, P = 0.67, ηp2 = 0.00. No effects of content and spatial position were observed for the P100.
DISCUSSION
In the present study, we investigated how fear modulates afferent sensory processing in lower-tier visual cortex. Searching for fear-specific and -unspecific objects in visual space, participants with high fear of specific objects (compared with non-fearful subjects) reliably showed enhanced amplitudes of the C1, the earliest component of visual processing in V1. This C1 modulation was not specifically related to the feared object but was shown for all target stimuli. These results demonstrate a top–down driven, heightened sensitivity to visual stimuli in the environment irrespective of their specific content. Although not discriminating between threat and non-threat stimuli, such sensory hypervigilance in high-fearful individuals appears to be evident already on the afferent pathway to the V1 in a situation in which confrontation with the feared object is expected.
Paralleling a large body of previous work, an enhanced C1 ERP component was detected with a typical widespread distribution over parieto-occipital scalp sites starting at ∼40 ms after stimulus onset. The C1 component is known as the first robust visual cortical response, originating in the striate cortex and therefore exclusively reflects V1 activity (e.g. Clark et al., 1995; Di Russo et al., 2002; Kelly et al., 2008). In accordance with the topographical distribution, the timing was also similar to recent visual selective attention and perception studies in humans and animals (Celebrini et al., 1993; Maunsell et al., 1999; Kelly et al., 2008; Rauss et al., 2011; West et al., 2011).
Although the C1 component was long interpreted as robust against cognitive top–down processes, the results of the present study are in line with recent findings that report modulations of the earliest measurable visual response by learning (e.g. Bao et al., 2010), affective meaning (Pourtois et al., 2004; Stolarova et al., 2006) and spatial attention (e.g. Kelly et al., 2008). Two main results were observed in the present study: First, the early part of the C1 component was influenced by the spatial location of visually presented target objects in high-fearful participants only, and second, the C1 was overall significantly enhanced in individuals with high fear of specific objects, indicating an enhanced endogenous vigilance signal in the V1.
Interestingly, the enhanced activity in V1 in the high-fearful group was not fear specific but enhanced for all contents, suggesting general visual hypervigilance for this group. Interestingly, Kolassa et al. (2007, Figure 5) found a similar ERP negativity ∼50 ms over occipital sensors. Although not explicitly tested, this negativity was enhanced for spider aficionados and spider phobics compared to controls, corroborating the present findings. Differences in the C1 component cannot be attributed to non-specific electrophysiological between-group differences, because spider-fearful participants were not different from non-anxious participants for the subsequent P100 component1 (100–120 ms). In contrast to the C1, the P1 was not affected by spatial position or any target content. These findings give rise to the assumption that specific fear modulates the initial V1 afference in an unspecific manner. That is, whenever there is the possibility that a fear-relevant stimulus might occur in the visual field, fearful participants show increased sensitivity to all displayed arrays, which in turn could amplify signals in early visual cortex. Anticipatory priming of V1 neurons has also been found in earlier fMRI studies, where sustained spatial attention activity was found in V1 in the absence of visual stimulation (Kastner et al., 1999; Silver et al., 2007). Recent visual imagery studies also reported increased activity in early visual cortex (Kosslyn and Thompson, 2003; review). Anticipation of feared pictures were associated with enhanced activity in extrastriate visual cortex (Straube et al., 2007), indicating enhanced visual attention while expecting the occurrence of fear-relevant stimuli. In the present study, increased neural activity is already evident in V1 neurons at the earliest cortical stage in high-fearful individuals.
Feedback signals from areas outside visual cortex are assumed to tune V1 neurons in primary visual cortex (e.g. Kastner et al., 1999; Rauss et al., 2011). It is possible that task-related top–down control from frontal and parietal cortex areas, known as part of spatial attention networks (e.g. Mohanty et al., 2009), is able to bias the incoming visual afference in V1. The finding that C1 amplitudes were more pronounced in the high-fear group might indicate enhanced frontal cortex-mediated top–down feedback to facilitate the rapid detection of aversive and fear-specific stimuli. Several speculative accounts are possible to explain such a top–down influence, including predictive coding (Rao and Ballard, 1999; Rauss et al., 2011), where incoming neural signals are matched with internal goals or predictions (prefrontal cortex) of occurring visual stimuli (e.g. the spider) by means of selective attention.
Interestingly, although behavioral performance did not differ between both groups, larger C1 amplitudes were associated with higher accuracy in high-fearful participants, indicating a state of hypervigilance in this group. General hypervigilance in highly fearful individuals (e.g. Gerdes et al., 2008) as shown in the present study is in line with the defense cascade model (Fanselow, 1994; Lang et al., 1997), in which general hypervigilance to all cues in a potentially dangerous context is considered to be the first step of defensive activation followed by selective attention to imminent threat once it occurs and defensive action (‘circa-strike’) if the threat is becoming more imminent. Once the organism is expecting potential threat, ‘pre-encounter’ defense is characterized by initial hypervigilance to all stimuli (as already visible in the C1 component), followed by a shift of attention toward the threat cue (‘post-encounter’ defense) when the threatening stimulus is detected. In line with this model, fear-specific modulations seem to occur at only later cortical stages as reported recently for the N1, N2pc, early posterior negativity (EPN) and late positive potentials (LPP) component (e.g. Kolassa et al., 2005; Leutgeb et al., 2009; Michalowski et al., 2009; Weymar et al., 2013).
Two open questions remain: First, is the enhanced V1 processing in fearful participants driven by general hypervigilance in everyday life or specific to a threat context, where the occurrence of a fearful stimulus is very likely? Because stimuli in the present experiment were shown in a randomized fashion, and trials with spiders occurred already at the beginning of the experiment, we cannot test this hypothesis using the existing data. However, a recent block design study by J.M. Michalowski et al. (personal communication) suggests that unspecific hypervigilance only occurs in fearful participants when the feared object is imminent. Investigating the P100, these authors found that in the first block of pictures where no spider was shown, P100 amplitudes were not different for neutral stimuli between spider-fearful participants and controls. However, when the same neutral stimuli were presented in blocks that also included picture of spiders, larger P100 amplitudes were observed for spider-fearful participants than controls, and this difference remained even later in the experiment when no spider was shown, indicating that unspecific hypervigilance in specific phobia occurs in contexts where the probability is high being confronted with their specific threat.
The second question arising from the present electrophysiological findings relates to the extent to which the sensory hypervigilance in spider-fearful participants is reflective of generally higher anxiety or of specific phobia. We collected questionnaire data2 [Trait scales of the German version of the Spielberger State-Trait Anxiety Inventory (STAI), Laux et al., 1981] and found that C1 amplitudes (40–60 ms) were still larger for spider-fearful individuals than non-fearful controls when both groups were matched based on the STAI trait scores. This suggests that the heightened sensory sensitivity (hypervigilance) in high-fearful individuals to the stimuli, whether fearful or not, is not eminent in individuals with heightened general trait-anxiety but more likely occurs in specific contexts they fear.
Taken together, the present results suggest that high specific fear already modulates the earliest stage of cortical processing in V1. Our findings suggest that incoming new visual information is already biased by threat-unspecific attention in high-fearful individuals.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Janine Wirkner for her assistance in data collection. This research was supported in part by a post-doctoral stipend from the German Research Society (Deutsche Forschungsgemeinschaft, DFG) to Mathias Weymar (Forschungsstipendium, WE 4801/1-1).
Footnotes
1Enhanced P1 amplitudes were previously found for spider-fearful and (socially) anxious participants relative to controls (e.g. Kolassa et al., 2006, 2007; Michalowski et al., 2009; Wieser et al., 2010) but not in the present study. However, the type of task (visual search) and stimulus material (either presented centrally or peripherally) might lead to study differences. In the present study, spider-fearful individuals showed larger amplitudes in the C1 and N1 (see Weymar et al., 2013) component relative to non-fearful individuals.
2To examine the possibility that hypervigilance in spider-fearful participants, as indexed by the enhanced C1 amplitudes, is attributed to general higher anxiety, we collected questionnaire data either before or after the experiment [Trait scales of the German version of the Spielberger State-Trait Anxiety Inventory (STAI), Laux et al., 1981] from 33 participants (16 spider fearful; 17 non-fearful). Trait anxiety was higher in the high-fear group (mean = 49.13) compared with low-fear group (mean = 40.53), F(1,31) = 12.86, P < 0.01. However, C1 amplitudes (40–60 ms) were still larger for spider-fearful individuals than non-fearful controls [F(1,18) = 7.88, P = 0.01, ηp2 = 0.30], when both groups were matched based on the STAI trait scores [spider fearful: Mean = 47.30; non-fearful: mean = 44.50; F(1,18) = 2.33, P = 0.14, ηp2 = 0.12]. This suggests that the heightened sensory sensitivity (hypervigilance) in high-fearful individuals to the stimuli, whether fearful or not, is related to the specific fear rather than heightened general anxiety.
REFERENCES
- Bao M, Yang L, Rios C, He B, Engel SA. Perceptual learning increases the strength of the earliest signals in visual cortex. Journal of Neuroscience. 2010;30:15080–4. doi: 10.1523/JNEUROSCI.5703-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: a metaanalytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Sutter EE. M and P components of the VEP and their visual field distribution. Vision Research. 1997;37:675–90. doi: 10.1016/s0042-6989(96)00209-x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G, Greenberg RL. Anxiety Disorders and Phobias: A Cognitive Perspective. New York: Basic Books; 1985. [Google Scholar]
- Byrne A, Eysenck MW. Trait anxiety, anxious mood, and threat detection. Cognition and Emotion. 1995;9:549–62. [Google Scholar]
- Celebrini S, Thorpe S, Trotter Y, Imbert M. Dynamics of orientation coding in area V1 of the awake monkey. Visual Neuroscience. 1993;10:811–25. doi: 10.1017/s0952523800006052. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional bias towards threat in anxiety disorders: an integrative review. Clinical Psychology Review. 2010;30:203–16. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Human Brain Mapping. 1995;2:170–87. [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Human Brain Mapping. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW. Anxiety: The Cognitive Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin and Review. 1994;1:429–38. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition and Emotion. 2002;16:355–79. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M. Reliability and validity of some specific fear questionnaires. Scandinavian Journal of Psychology. 1983;24:331–4. doi: 10.1111/j.1467-9450.1983.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Gerdes ABM, Alpers GW, Pauli P. When spiders appear suddenly: Spider phobic patients are distracted by task-irrelevant spiders. Behavior Research and Therapy. 2008;46:174–87. doi: 10.1016/j.brat.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Gerdes ABM, Uhl G, Alpers GW. Spiders are special: fear and disgust evoked by pictures of arthropods. Evolution and Human Behavior. 2009;30:66–73. [Google Scholar]
- Gilboa-Schechtman E, Foa EB, Amir N. Attentional biases for facial expressions in social phobia: The face-in-the-crowd paradigm. Cognition and Emotion. 1999;13:305–18. [Google Scholar]
- Hamm AO. 2006. Spezifische Phobien [Specific Phobias]. Göttingen: Hogrefe Verlag. [Google Scholar]
- Holmes A, Bradley BP, Kragh Nielsen M, Mogg K. Attentional selectivity for emotional faces: evidence from human electrophysiology. Psychophysiology. 2009;46:62–8. doi: 10.1111/j.1469-8986.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA, Axford JG. Source locations of pattern-specific components of human visual evoked potentials: I. Component of striate cortical origin. Experimental Brain Research. 1972;16:1–21. doi: 10.1007/BF00233371. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker D, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–32. [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–61. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Ihssen N, et al. Defensive engagement and perceptual enhancement. Neuropsychologia. 2010;48(12):3580–4. doi: 10.1016/j.neuropsychologia.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial attention modulates initial afferent activity in human primary visual cortex. Cerebral Cortex. 2008;18:2629–36. doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. Psychometric description of some specific fear questionnaires. Behavior Therapy. 1974;5:401–9. [Google Scholar]
- Kolassa I-T, Buchmann A, Lauche R, Kolassa S, Miltner WHR, Musial F. Spider phobics more easily see a spider in morphed schematic pictures. Behavioral and Brain Functions. 2007;3:59. doi: 10.1186/1744-9081-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I-T, Musial F, Kolassa S, Miltner WHR. Event-related potentials when identifying or color-naming threatening schematic stimuli in spider phobic and non-phobic individuals. BMC Psychiatry. 2006;6:38. doi: 10.1186/1471-244X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa IT, Musial F, Mohr A, Trippe RH, Miltner WHR. Electrophysiological correlates of threat processing in spider phobics. Psychophysiology. 2005;42:520–30. doi: 10.1111/j.1469-8986.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL. When is early visual cortex activated during visual mental imagery? Psychological Bulletin. 2003;129:723–46. doi: 10.1037/0033-2909.129.5.723. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert MM. Motivated attention: affect, activation and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. 1997. pp. 97–135. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger CD. 1981. Das State-Trait-Angstinventar (STAI) [State-Trait Anxiety Inventory (STAI)]. Weinheim: Beltz Test. [Google Scholar]
- Leutgeb V, Schäfer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology. 2009;82:293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, et al. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–9. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Ghose GM, Assad JA, McAdams CJ, Boudreau CE, Noerager BD. Visual response latencies of magnocellular and parvocellular LGN neurons in macaque monkeys. Visual Neuroscience. 1999;16:1–14. doi: 10.1017/s0952523899156177. [DOI] [PubMed] [Google Scholar]
- Michalowski JM, Melzig CA, Weike AI, Stockburger J, Schupp HT, Hamm AO. Brain dynamics in spider-phobic individuals exposed to phobia-relevant and other emotional stimuli. Emotion. 2009;9:306–15. doi: 10.1037/a0015550. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neuroscience and Biobehavioral Reviews. 2012;36:459–78. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley B. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–48. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Egner T, Monti JM, Mesulam MM. Search for a threatening target triggers limbic guidance of spatial attention. Journal of Neuroscience. 2009;29:10563–72. doi: 10.1523/JNEUROSCI.1170-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Herrmann MJ, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher social anxious persons. Journal of Neural Transmission. 2009;116:735–46. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H. A comparison of two spider fear questionnaires. Journal of Behavior Therapy and Experimental Psychiatry. 1996;27:241–4. doi: 10.1016/s0005-7916(96)00022-5. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–78. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Parker DM, Salzen EA, Lishman JR. The early wave of the visual evoked potential to sinusoidal gratings: responses to quadrant stimulation as a function of spatial frequency. Electroencephalography and Clinical Neurophysiology. 1982;53:427–35. doi: 10.1016/0013-4694(82)90007-4. [DOI] [PubMed] [Google Scholar]
- Peyk P, De Cesarei A, Junghöfer M. Electro Magneto Encephalography Software: overview and integration with other EEG/MEG toolboxes. Computational Intelligence and Neuroscience. 2011;2011:861705. doi: 10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugshaupt T, Mosimann UP, von Wartburg R, Schmitt W, Nyffeler T, Müri RM. Hypervigilance-avoidance pattern in spider phobia. Journal of Anxiety Disorders. 2005;19:105–16. doi: 10.1016/j.janxdis.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–33. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Rao RPN, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nature Neuroscience. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Rauss KS, Pourtois G, Vuilleumier P, Schwartz S. Attentional load modifies early activity in human primary visual cortex. Human Brain Mapping. 2009;30:1723–33. doi: 10.1002/hbm.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauss K, Schwartz S, Pourtois G. Top-down effects on early visual processing in humans: A predictive coding framework. Neuroscience and Biobehavioral Reviews. 2011;35:1237–53. doi: 10.1016/j.neubiorev.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York: Elsevier; 1989. [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Neural correlates of sustained spatial attention in human early visual cortex. Journal of Neurophysiology. 2007;97:229–37. doi: 10.1152/jn.00677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spekreijse H, van der Twell LH, Zuidema T. Contrast evoked responses in man. Vision Research. 1973;13:1577–601. doi: 10.1016/0042-6989(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cerebral Cortex. 2006;16:876–87. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- West GL, Anderson AAK, Ferber S, Pratt J. Electrophysiological evidence for biased competition in V1 for fear expressions. Journal of Cognitive Neuroscience. 2011;23:3410–18. doi: 10.1162/jocn.2011.21605. [DOI] [PubMed] [Google Scholar]
- Weymar M, Gerdes ABM, Löw A, Alpers GW, Hamm AO. Specific fear modulates attentional selectivity during visual search: Electrophysiological insights from the N2pc. Psychophysiology. 2013;50:139–48. doi: 10.1111/psyp.12008. [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Öhman A, Hamm AO. The face is more than its parts – Brain dynamics of enhanced spatial attention to spatial threat. Neuroimage. 2011;58:946–54. doi: 10.1016/j.neuroimage.2011.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Reicherts P, Mühlberger A. Don't look at Me in Anger! - enhanced processing of angry faces in anticipation of public speaking. Psychophysiology. 2010;47:271–80. doi: 10.1111/j.1469-8986.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention: a review of attentional processing of emotional information. Cognition and Emotion. 2010;24:3–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.