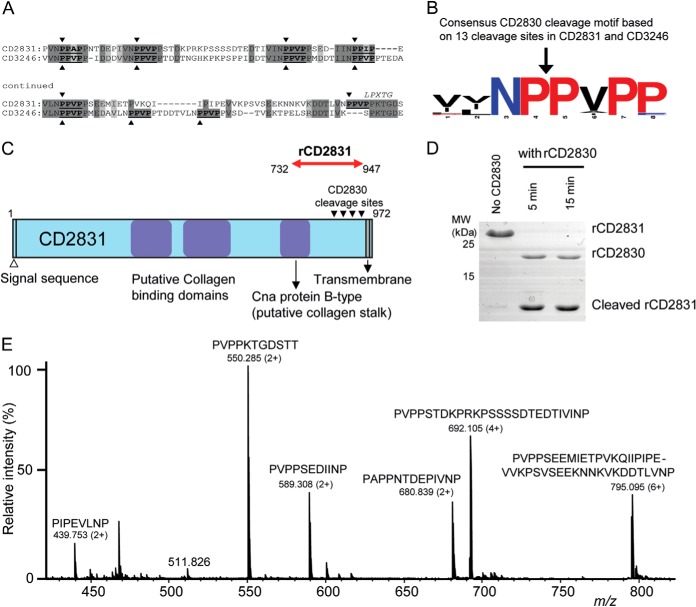
Fig. 4.
Identification of multiple CD2830 cleavage sites in putative C. difficile surface proteins. A, identification of multiple CD2830 cleavage sites (indicated by arrowheads) in the C-terminal region of the C. difficile putative adhesion proteins CD2831 and CD3246. Both putative substrates contain an LPXTG motif (PPXTG and SPXTG, respectively) and show considerable sequence similarity around the CD2830 cleavage sites. B, prevalence of amino acids around the scissile bond in the 13 CD2830 cleavage sites identified in CD2831 and CD3246. The cleavage sites were aligned using WebLogo and visualized using the following coloring: red, proline; black, nonpolar; blue, polar (not proline). C, schematic representation of C. difficile CD2831 showing the putative collagen binding domains and transmembrane domain. Recombinant CD2831 (rCD2831) protein corresponding to amino acids 732–947 (red arrow) was produced containing all the CD2830 cleavage sites. D, SDS-PAGE analysis of rCD2831 treated with rCD2830 for different time periods. E, mass spectrometric analysis (Q-TOF-MS) of the small peptide cleavage products derived from rCD2831 after incubation with rCD2830 for 30 min.