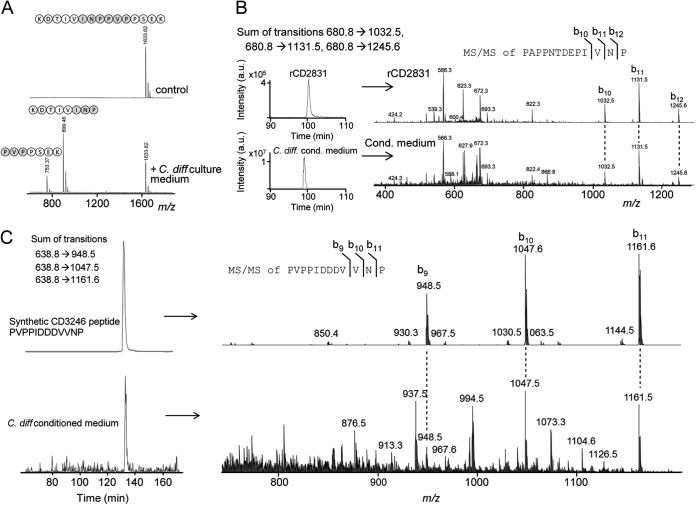
Fig. 5.
Detection of native CD2830 protease activity in the secretome of C. difficile and cleavage of endogenous CD2831 and CD3246. A, a synthetic peptide containing one of the CD2830 cleavage sites in CD2831 (KDTIVINPPVPPSEK) was incubated with the conditioned medium collected from cultures of C. difficile cells. After incubation for 16 h, samples were analyzed via MALDI-TOF-MS to determine substrate peptide cleavage. B, upper panel: LC–ion trap MS/MS analysis of recombinant CD2831 after treatment with rCD2830 demonstrating the elution profile (left) and MS/MS identification (right) of the CD2831 cleavage product PAPPNTDEPIVNP. Lower panel: LC-MS/MS analysis of C. difficile conditioned medium. Shown are the sum of the specific transitions 680.8 → 1032.5, 680.8 → 1131.5, and 680.8 → 1245.6 in the LC-MS/MS elution profile (left) and MS/MS identification (right), which are both equivalent to that observed with rCD2831 (upper panel), thereby unambiguously demonstrating the presence of the CD2831 cleavage product in C. difficile conditioned medium. C, upper panel: a synthetic peptide corresponding to one of the putative cleavage products of CD3246 (PVPPIDDDVVNP) was analyzed via LC–ion trap MS/MS to determine its elution time and MS/MS spectrum. Lower graph: LC-MS/MS analysis of C. difficile conditioned medium. Shown are the sum of the specific transitions 638.8 → 948.5, 638.8 → 1047.6, and 638.8 → 1161.6 (left) and MS/MS identification (right), which are both equivalent to that observed with the synthetic peptide (upper panel), thereby clearly demonstrating the presence of the CD3246 cleavage product in C. difficile conditioned medium.