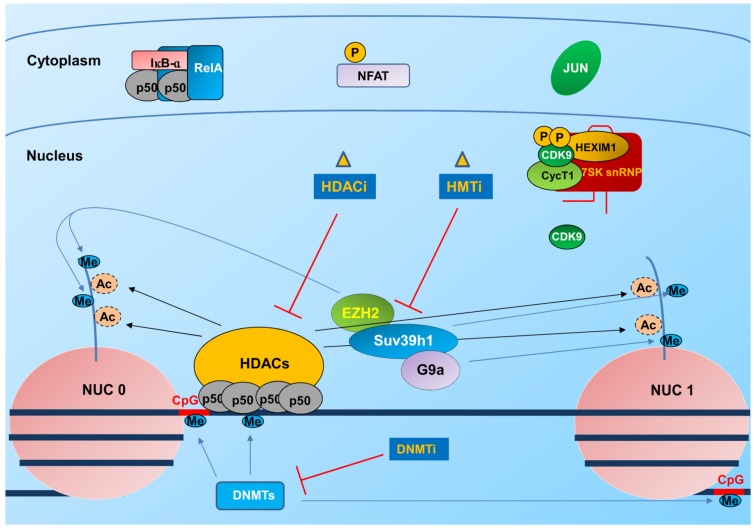
Figure 2.
HIV-1 gene transcription is silenced in latently infected cells: epigenetic mechanisms of silencing affected by epigenetic modulators. Transcription initiation at the HIV-1 LTR is inhibited in latently infected CD4+ T cells due to different epigenetic silencing mechanisms. These include: recruitment of histone deacetylases (HDACs) by the NF-κB p50/p50 homodimer resulting in deacetylation of histones at the Nuc0 and Nuc1 nucleosomes, recruitment of histone methyltransferases (HMTs) as Suv39h1, EZH2 and G9a, resulting in methylation of histones and DNA methyltransferases responsible for DNA methylation at CpG islands. Crucial transcription factors responsible for initiating transcription at the LTR, such as NF-κB, NFAT and cJun (a sub unit of AP1) are then sequestered in the cytoplasm in an inactive state, contributing in the establishment/maintenance of latency. The P-TEFb factor, crucial for HIV-1 transcriptional elongation, is part of an inactive complex and together with the low amounts of the P-TEFb subunit cyclin T1 in latently infected CD4+ T cells, represent a further mechanism of transcriptional restriction. HDAC, HMT and DNMT inhibitors are all being explored to promote escape from latency in the context of the “shock/kick and kill” strategy.