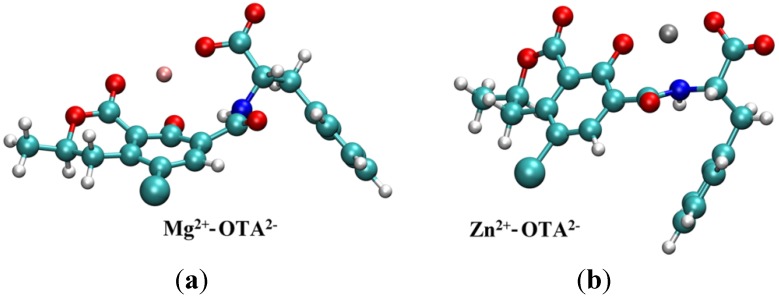
Figure 4.
(a) [Mg2+-OTA2−] structure. The Mg2+ ion primarily interacts with three partially negatively charged oxygen atoms. Based on NBO analysis as implemented in the Gaussian program, the total charge on Mg2+ is 1.65 e, while there are −0.99 e, −0.87 e and −0.78 e charges on the three oxygen atoms (one in the carboxyl group, the phenolic oxygen and the oxo group). The amide nitrogen possesses a −0.63 e charge compared to the −0.64 e charge in the free OTA2−; (b) [Zn2+-OTA2−] structure. The Zn2+ ion can be found close to the two partially negatively charged oxygen atoms and to the nitrogen atom of the amide group. The total charge on Zn2+ is 1.38 e, while there are −0.83 e and −0.81 e charges on the two oxygen atoms (carboxyl and phenolic, respectively) and −0.73 e on the nitrogen atom.