Figure 2.
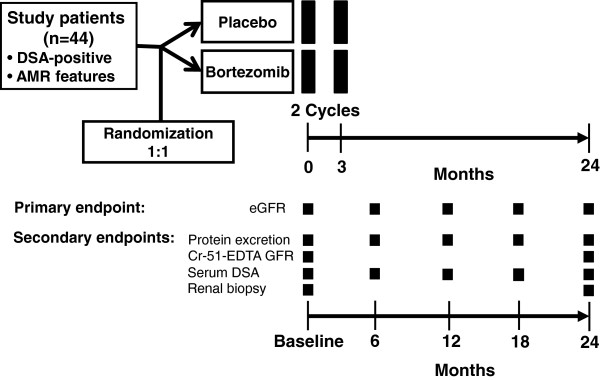
Study flowchart of Part B (randomized controlled trial). Forty-four transplant recipients with late biopsy-proven antibody-mediated rejection (AMR) will be randomized to receive either bortezomib or placebo. The primary endpoint, the estimated glomerular filtration rate (eGFR), will be evaluated at 0, 6, 12, 18 and 24 months. Major secondary endpoints are the measured glomerular filtration rate (GFR), protein excretion, patterns of human leukocyte antigen reactivity and results obtained with 24-month protocol biopsies. Cr-EDTA, chromium ethylenediamine tetraacetic acid; DSA, donor-specific antibodies; MFI, mean fluorescence intensity.
