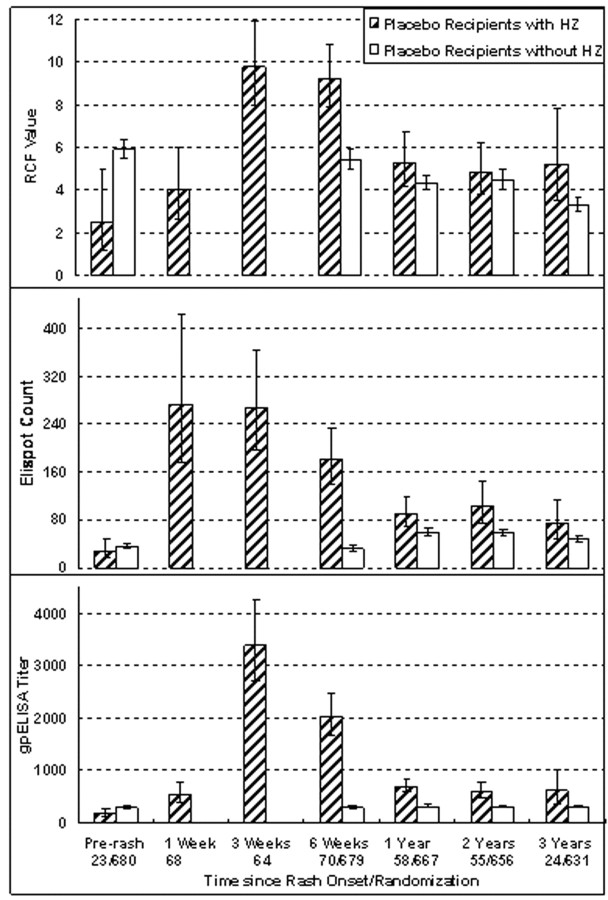
Figure 5.
Comparison of varicella-zoster virus (VZV)–specific immune responses of placebo recipients who developed herpes zoster (HZ) with placebo recipients who did not develop HZ. Data were derived from 73 subjects who received placebo at clinical sites where an immunology laboratory (IL) was located, developed HZ, and were followed up at those sites, as well as from 680 placebo recipients without HZ who were enrolled in the Immunology Substudy and were also followed up at the IL sites. Bars indicate geometric means and 95% confidence intervals (CIs) for the absolute responder cell frequency (RCF) values, measured as responder cells per 105 peripheral blood mononuclear cells (PBMCs); enzyme-linked immunospot (ELISPOT) counts, measured as spot-forming cells per 106 PBMCs; and titers for enzyme-linked immunosorbent assay (ELISA) against affinity-purified VZV glycoproteins (gpELISA), measured as ELISA units per milliliter. Levels before rash onset for subjects without HZ were those measured at enrollment. Levels at other time points were measured after onset of HZ rash in the subjects with HZ or after enrollment in the subjects without HZ. Numbers indicate the numbers of subjects contributing samples at each time point. Subjects who developed HZ had significantly lower values for RCF (P=.001) and gpELISA titers (P=.02) but not for ELISPOT counts at the last visit before HZ onset, compared with subjects who did not develop HZ. HZ significantly increased all immune responses