Abstract
During the past 10 years, a global pandemic of end‐stage renal disease (ESRD) attributed to diabetes mellitus has changed the therapeutic strategies based on landmark trials that have shown that diabetic micro‐ and macrovascular complications might be preventable. However, the remaining risk of the progression of diabetic kidney disease to ESRD is still high, despite newly introduced anti‐diabetic, antihypertensive and dyslipidemic drugs in the 21st century. Here, we show the importance of targeting remission and regression of microalbuminuria in type 2 diabetic patients. To achieve the remission and regression of microalbuminuria, physicians have revised the management strategy of diabetic patients and have to act immediately. Early detection of microalbuminuria with continuous screening, the use of renin–angiotensin system blockades, and targets for HbA1c of <7.35% and systolic blood pressure of <130 mmHg are closely associated with the remission and regression of microalbuminuria, resulting in protection against the progression of diabetic kidney disease, as well as cardiovascular events. Our concept of the natural history of diabetic kidney disease has to be modified by our results and others. Reducing microalbuminuria is therefore considered to be an important therapeutic target and could be a pivotal biomarker of therapeutic success in diabetic patients. (J Diabetes Invest, doi:10.1111/j.2040‐1124.2011.00112.x, 2011)
Keywords: Remission, Microalbuminuria, Overt proteinuria
Introduction
The persistent rise in the proportion of chronic dialysis patients resulting from diabetic kidney disease in Asian countries, including Japan, over the past 20 years has been associated with higher mortality and is widely recognized as a major public health concern1. To combat this problem, intensive efforts are underway to clarify the evolving management contributing to the amelioration of the development and progression of diabetic kidney disease. Amongst newly introduced anti‐diabetic agents, inhibitors of the renin–angiotensin system (RAS) with either angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), peroxisome proliferator‐activated receptor (PPAR) alpha agonist and, recently, paricalcitol have been reported to prevent the development and progression of diabetic kidney disease2–7. However, the residual risk still remains high8. In this review, we focus on the reason why a global pandemic of end‐stage renal disease (ESRD) attributed to diabetes has continued, as well as how we can challenge diabetic kidney disease in clinical practice.
Epidemiology
The World Health Organization (WHO) estimated that more than 300 million people will have diabetes by 20259, resulting in a pandemic that threatens to collapse socioeconomic recourses. More recently, the Baker IDI Heart and Diabetes Institute estimated that the world prevalence of diabetes among adults (aged 20–79 years) will increase to 7.7%, and 439 million adults by 203010. Similarly, the number of people who have diabetes or who are suspected of having diabetes has increased by 1.6‐fold over the past decade, and this trend is suspected to continue in Japan.
As a result, the number of people who have diabetic kidney disease has increased and diabetic ESRD, in particular, has been the main cause of newly introduced diseases to chronic dialysis since 1998, and the trend is still continuing in Japan1. In contrast, although the number of new cases of ESRD with diabetes has increased, the rate of new cases of ESRD requiring dialysis among Americans diagnosed with diabetes fell 35% between 1996 and 2007, according to a study by the US Centers for Disease Control and Prevention11. Here, we raise several reasons to explain the discrepancy between Asian and Western countries (Table 1). Increased numbers of patients with diabetes; patients’ higher ignorance when receiving treatment; poorly controlled blood glucose, blood pressure and lipids; lower rate of preventable screening for indication of developing diabetic kidney disease; and the aging process could contribute to an increased rate of new cases of ESRD requiring dialysis in Japan. As compared with Western countries, the proportion of patients with diabetes receiving a recommended medical evaluation, such as an annual urinary albumin measurement, has increased from 21.7% in 2000 to 27.2% in 2006 in the Shiga Prefecture, Japan. Furthermore, even in hospitals and clinics where diabetes specialists were taking care of patients, almost half of the diabetic patients were not receiving the measurement of urinary albumin from January 2004 to July 200512. In other words, the measurement of urinary albumin is still neglected in clinical practice, although many clinicians know the importance of albuminuria as an indicator of diabetic kidney disease as well as a sensitive, accessible predictor of cardiovascular risk. In addition, ESRD in Japan has been regarded as a geriatric disorder and the mean age of newly diagnosed ESRD patients with diabetes was 2.5 years older than that in the USA (mean age 65.7 years in Japan vs 63.2 year in the USA)1,13, suggesting that the aging process could also contribute to an increased incidence of diabetic ESRD in Japan.
Table 1. Issues in addressing a global pandemic of end stage renal disease attributed to diabetes in Japan.
| Increased numbers of patients with diabetes or suspected of having diabetes |
| Patients’ higher ignorance receiving diabetes treatment |
| Poorly controlled blood glucose, blood pressure and lipids |
| Lower rate of screening for indication of developing diabetic kidney disease |
| Aging process |
Screening methods for diabetic nephropathy
The earliest clinical sign of diabetic kidney disease is an elevated urinary albumin excretion, referred to as microalbuminuria14,15. Microalbuminuria is defined as an albumin excretion rate (AER) of 20–199 μg/min in a timed or a 24‐h urine collection (equivalent to urinary albumin creatinine ratio [ACR] of 30–299 mg/g creatinine in a random spot sample). Microalbuminuria progresses to overt proteinuria, leading to a decline in renal function defined as glomerular filtration rate (GFR)14. Generally, overt proteinuria inexorably progresses to ESRD 6–8 years after the detection of overt proteinuria15. Thus, microalbuminuria in diabetic patients has been recognized as a predictor of progression to ESRD. Based on the data from over 5000 patients who were followed from the first diagnosis of type 2 diabetes in The United Kingdom Prospective Diabetes Study (UKPDS), annual transition rates from one stage to another stage of diabetic kidney disease were approximately 2% at each stage16. Furthermore, microalbuminuria has been shown to be closely associated with a higher risk for cardiovascular morbidity and mortality17–19. Indeed, the cardiovascular mortality in type 2 diabetic patients with microalbuminuria has been reported to be twofold higher than that in patients with normoalbuminuria16. Therefore, microalbuminuria is an important therapeutic target to improve the prognosis of renal and cardiovascular risk in diabetic patients.
Therapeutic strategy of diabetic kidney disease
Targeting euglycemia
Based on landmark clinical trials, intensive regimens of glucose control have been shown to reduce the development and progression of diabetic kidney disease20–22. Furthermore, the persistence of microvascular benefits in patients, who were previously intensively treated, was reported in the follow‐up study of The Diabetes Control and Complications Trial (DCCT) in the Epidemiology of Diabetes Interventions and Complications (EDIC) and of the UKPDS, although their glycemic control has been equivalent to that of previous control arm subjects during follow up23–25.
Recent trials in patients with more long‐standing type 2 diabetes have also confirmed the benefit of intensifying glucose control on development and/or progression of microvascular complications, including diabetic kidney disease. The Veterans Affairs Diabetes Trial (VADT) showed significant reductions in albuminuria with intensive glycemic control (achieved median HbA1c 6.9%) compared with standard glycemic control, although intensifying glucose control failed to affect other primary and secondary end‐points beneficially26,27. The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial has also shown that intensifying glucose control to achieve a HbA1c level of <6.5% did provide the benefit of reducing the risk of both the development and progression of diabetic kidney disease28. As compared with standard control, intensive control was associated with a significant reduction in new‐onset microalbuminuria by 9%. Furthermore, intensifying glucose control resulted in a significant reduction in renal events by 21%, including new or worsening diabetic kidney disease defined as the development of overt proteinuria, renal replacement therapy or death from renal causes, although the incidence of the doubling of serum creatinine level did not differ. Unfortunately, this study also failed to reduce the incidence of major macrovascular events defined as myocardial infarction, stroke or cardiovascular death with intensive control as compared with standard control. Nevertheless, the reduction in the incidence of diabetic kidney disease might have long term benefits on cardiovascular disease, because diabetic patients with kidney disease have a higher risk of macrovascular disease. However, the results of Action to Control Cardiovascular Risk in Diabetes (ACCORD) showed that the risk of death was increased by near‐normal glycemic control with intensifying treatment as compared with standard control without the reduction of cardiovascular events29, although recent analyses from the ACCORD trial have shown that the risk of development of overt proteinuria was 31% lower with intensive therapy at transition and 28% lower at study end than with standard therapy30. As reported in the post‐hoc epidemiological analysis of the ACCORD study31, we need to pay attention to what the benefit of intensifying glucose control for diabetic patients is. It must be weighed against the risks of intensive glycemic control, including all‐cause and cardiovascular disease‐related mortality, weight gain, and incidence of severe hypoglycemic episodes. Furthermore, a recent subanalysis of the ADVANCE trial clearly showed that severe hypoglycemia was strongly associated with an increased risk of a range of adverse clinical outcomes, including macrovascular and/or microvascular events as well as death32.
Intensive glycemic treatment targeting a HbA1c goal level of 6.0% or less could be beneficial for individuals who are younger and have newly diagnosed diabetes. However, a conservative HbA1c targeting the 7% range might be appropriate in older individuals who have established diabetes, cardiovascular disease and major risk factors for cardiovascular disease. Therefore, the goals for managing elderly patients with diabetes, especially type 2 diabetes, should be individualized according to the patient’s age, disease stage and other comorbid conditions. Indeed, the American Diabetes Association 2011 recommends a HbA1c level below or around 7% to reduce microvascular and macrovascular complications of diabetes in patients soon after the diagnosis of diabetes14.
Blood pressure control with RAS inhibitors
Strict blood pressure control of <130/80 mmHg is universally recommended in patients with diabetes to lower incidences of stroke, heart failure, diabetes‐related death, retinal photocoagulation and to reduce the risk of micro‐ or overt proteinuria. In the recent ADVANCE study, the reduction of blood pressure from 140/73 mmHg (control group) to 136/73 mmHg (indapamide–perindopril group) was shown to reduce the risks of a major macro‐ or microvascular (mostly new microalbuminuria) event, death from cardiovascular disease and death from any cause after 4.3 years of follow up33, extending the early findings of the UKPDS34 to an even lower blood pressure. Therefore, targeting blood pressure <130/80 mmHg appears to be appropriate in type 2 diabetics to fight against the development and progression of diabetic kidney disease35.
In diabetic patients with microalbuminuria or overt proteinuria, RAS inhibitors play a pivotal role in the prevention and treatment of diabetic kidney disease. Landmark studies with type 1 and type 2 diabetic patients at various stages of diabetic kidney diseases have well provided the clinical evidence that treatment with RAS inhibitors did slow the progressive decrease in GFR, reduce proteinuria and microalbuminuria, prevent progression from one stage of diabetic kidney disease to others, and reduce cardiovascular mortality and morbidity as shown in Figure 136–47. As described in the next section, recent studies have shown the effectiveness of ARB, not only to reduce the progression of diabetic kidney disease to ESRD, but also to revert the progressive course. Its achievement resulted in a long‐term stabilization of renal function and cardiovascular protection.
Figure 1.
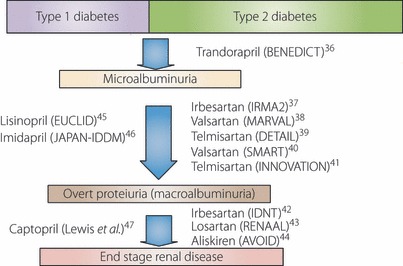
Landmark studies showing the effectiveness of renin–angiotensin system inhibitors on diabetic kidney disease, and cardiovascular mortality and morbidity.
Dual RAS blockade with ACEi and ARB might be more effective in reducing proteinuria compared with monotherapy in patients with diabetic kidney diseases. Based on the Ongoing Telmisartan Alone and in combination with the Ramipril Global Endpoint Trial (ONTARGET), although combination therapy with ramipril and telmisartan reduces proteinuria than monotherapy, it worsens major renal outcomes including dialysis, doubling of serum creatinine and death48,49. Thus, combination RAS blockade should not be used in diabetic patients, especially elderly type 2 diabetic patients with normo‐ and/or microalbuminuria. First, ACEi or ARB should be used and their dosage should be increased to obtain an optimal anti‐albuminuric and/or proteinuric response. Combination treatment with both ACEi and ARB should be prescribed by a nephrologist, and given to those patients with overt proteinuria and/or massive proteinuria despite the use of maximum dosages of ACEi or ARB. In those diabetic patients, monitoring of renal function is needed, and the treatment should be stopped in the event of acute kidney injury, low blood pressure and/or high potassium level. However, the effect of combination treatment with aliskiren and ARB in type 2 diabetic patients with overt diabetic kidney disease was recently reported44. In the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study, 599 patients with diabetic kidney disease with overt proteinuria were treated with losartan 100 mg, followed by the addition of a placebo or aliskiren (300 mg). As a result, treatment with 300 mg of aliskiren daily reduced the mean urinary ACR by 20% as compared with the placebo, with a reduction of 50% or more in 24.7% of the patients who received aliskiren as compared with 12.5% of those who received the placebo. At present, the Aliskiren Trial in Type 2 Diabetes Using Cardio‐Renal Endpoints (ALTITUDE) to confirm the effectiveness of combination treatment with either an ACEi or an ARB plus aliskiren on both renal and cardiovascular events is ongoing, in which diabetic patients with proteinuria and a history of cardiovascular disease were enrolled50.
Although RAS inhibitors have become the mainstays of treating established diabetic kidney disease51, the beneficial effects of these agents on the early phases of diabetic kidney disease is unclear. If hypertensive diabetic patients have normoalbuminuria, how should we treat them? Recent studies have reported the negative results of treatment with RAS inhibitors. Bilous et al.52 examined the effect of candesartan on microalbuminuria and albumin excretion rates, either before renal disease began or in its earliest stages based on data from the Diabetic Retinopathy Candesartan Trials (DIRECT) randomized trials. The incidence of microalbuminuria in type 1 diabetes was 5% in both the candesartan and placebo groups, and that of microalbuminuria in type 2 diabetics was 12% in the candesartan group compared with 13% in the placebo group. Candesartan failed to prevent microalbuminuria in these diabetic patients. Mann et al.53 also examined the long‐term renal effects of another ARB, telmisartan, in adults who were intolerant to ACEi, but had a high risk of vascular disease without albuminuria at baseline. Although treatment with telmisartan significantly reduced the risk for new microalbuminuria, overt proteinuria, or both, the reduction in albuminuria was not associated with less progression of renal disease, including dialysis or doubling of serum creatinine. Therefore, to decide whether we need to use RAS inhibitors in hypertensive and diabetic patients, the degree of the patient’s vascular and renal risk must be assessed in addition to taking into account the efficacy on the functions of the cardiovascular system and diabetic kidney disease.
Remission and regression of early stage of diabetic kidney disease and cardio‐renal protection
We carried out a prospective observational follow‐up study including a total of 216 Japanese type 2 diabetic patients with microalbuminuria54. In our study, we used the definition of remission/regression of microalbuminuria similar to that of the Perkins et al. study55. The remission was defined as a shift of the AER from microalbuminuria to normoalbuminuria, and the regression was defined as a 50% reduction in the AER from baseline. The 6‐year cumulative incidence of progression from microalbuminuria to overt proteinuria was 28% (95% CI 19–37), whereas those for remission and regression were 51% (95% CI 42–60) and 54% (95% CI 45–63), respectively (Figure 2). In the pooled logistic regression analysis, each modifiable factor was trisected according to the number of patients and was applied as three categories in the analysis. The results showed that microalbuminuria of short duration, the use of RAS blockades, a HbA1c level of <7.35%56 and lower systolic blood pressure <130 mmHg were identified to be independent factors associated with remission/regression of microalbuminuria. Angiotensin‐II receptor blockers have also been shown to induce remission and regression of microalbuminuria in Japanese type 2 diabetic patients40,41. In the Shiga Microalbuminuria Reduction Trial, 150 patients with microalbuminuria were randomly assigned to either the valsartan group or the amlodipine group and followed for 24 weeks. During the study, levels of blood pressure were similar in both groups. However, the frequency of patients who achieved remission or regression of microalbuminuria was significantly higher in the valsartan group than in the amlodipine group (remission 23 vs 11%, P = 0.011; regression 34 vs 16%, P = 0.008)40. In another Japanese Incipient to Overt: Angiotensin II Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) study, microalbuminuria remission at final observation occurred in 21.2% of patients with 80 mg of telmisartan, 12.8% of patients with 40 mg of telmisartan and 1.2% of patients with a placebo (both telmisartan doses vs placebo, P < 0.001)41. In addition, patients receiving 80 or 40 mg of telmisartan achieved superior renoprotection, shown by lower transition rates to overt nephropathy, compared with the placebo41. Taken together, these results strongly indicate that RAS blockade by using ARB not only prevent the progression of microalbuminuria to overt proteinuria, but also induce remission and regression of microalbuminuria in Japanese type 2 diabetic patients.
Figure 2.
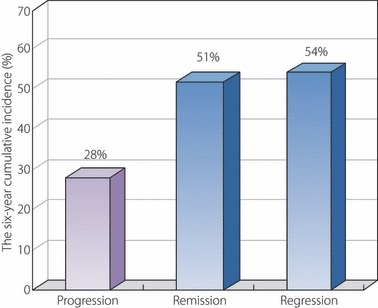
A prospective observational follow‐up study including a total of 216 Japanese type 2 diabetic patients with microalbuminuria was carried out to follow the change of stage of microalbuminuria for 6 years. The remission was defined as a shift of the albumin excretion rate (AER) from microalbuminuria to normoalbuminuria, and the regression was defined as 50% reduction in the AER from baseline.
Similar to ours, the Steno‐2 study also reported that a high proportion of patients with microalbuminuria returned to normoalbuminuria with a multifactorial intervention in 151 type 2 diabetic patients with microalbuminuria57. After a mean of 7.8 years of follow up, 46 (31%) patients returned to normoalbuminuria, 58 (38%) patients still had microalbuminuria and 47 (31%) patients progressed to overt proteinuria. Lower HbA1c, starting antihypertensive therapy and starting RAS inhibitor drugs during the follow up were independently associated with the remission of microalbuminuria. Recent analysis, especially regarding the effect of lowering blood pressure, clearly showed that more than half of all type 2 diabetic patients with microalbuminuria and macroalbuminuria returned to normoalbuminuria with any blood pressure lowering drugs in the ADVANCE study58. However, more patients assigned to perindopril–indapamide treatment than those assigned to placebo treatment achieved remission to normoalbuminuria58.
To explore the clinical impact of a reduction of microalbuminuria, we expanded the follow up by a 2 years beyond our previous study54 and examined whether remission and regression of microalbuminuria could translate into risk reduction of renal and cardiovascular events59. The primary evaluation consisted of combined incidence defined as cardiovascular death, and first hospitalization for renal and cardiovascular events. A secondary evaluation was the kidney function as determined by the annual decline rates of estimated GFR (eGFR). During the 8‐year follow‐up period, a total of 47 patients experienced primary renal and cardiovascular events. The number of first occurrences of outcomes in subgroups, who achieved remission of microalbuminuria, was 11 events and 36 events in the non‐remission group. The pooled logistic analysis adjusted by sex, age, the initial AER levels, a history of cardiovascular disease, current smoking, HbA1c, total cholesterol, blood pressure, the use of RAS inhibitors, the use of lipid lowering drugs and body mass index (BMI) showed that the risk for outcomes in patients, who achieved remission, was 0.25 (95% CI 0.07–0.87) as compared with those whose microalbuminuric stage did not change during the follow up, whereas that in patients who progressed to overt proteinuria was 2.55 (95% CI 1.04–6.30) (Figure 3). Even though failing to achieve the remission, the number of the first occurrences of outcomes in subgroups stratified by a 50% reduction of urinary albumin excretion was 12 events in the regression group and 35 events in the non‐regression group. The Kaplan–Meier estimation showed that the cumulative incidence of evaluated events was significantly lower in the regression group than in the non‐regression group. The 8‐year cumulative incidence of these outcomes in the regression group showed a 59% decrease compared with the non‐regression group. The adjusted risk for outcomes in patients who achieved the regression was 0.41 (95% CI 0.15–0.96) as compared with those whose microalbuminuric stage did not achieve the regression during the follow up.
Figure 3.
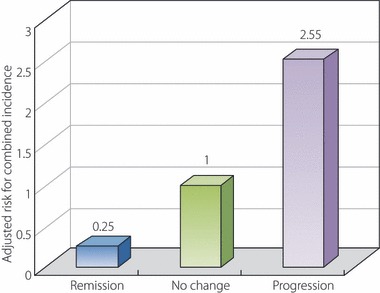
A prospective observational follow‐up study including a total of 216 Japanese type 2 diabetic patients with microalbuminuria was carried out to explore the clinical impact of remission and progression of microalbuminuria. The primary evaluation consisted of combined incidence defined as cardiovascular death and first hospitalization for renal and cardiovascular events. The pooled logistic analysis adjusted by sex, age, the initial albumin excretion rate levels, a history of cardiovascular disease, current smoking, HbA1c, total cholesterol, triglyceride, high‐density lipoprotein cholesterol, systolic blood pressure, diastolic blood pressure, the use of renin–angiotensin system inhibitors, the use of lipid lowering drugs and body mass index showed that the risk for outcomes in patients, who achieved remission, was 0.25 (95% CI 0.07–0.87) as compared with those whose microalbuminuric stage did not change during the follow up, whereas that in patients, who progressed to overt proteinuria, was 2.55 (95% CI 1.04–6.30).
As suspected, the annual decline rate of eGFR in the progression group (median −4.2 mL/min/year) was significantly faster than in the non‐change group (−2.4 mL/min/year), whereas the annual decline rate of eGFR in the remission group was significantly slower, −1.1 mL/min/year, which is almost identical with the decline rate by normal aging reported in healthy people60. The effect of reducing microalbuminuria on kidney function was also reported in the Steno‐2 study aforementioned57. The patients who reverted to normoalbuminuria had an eGFR decline of 2.3 mL/min/year; however, those who still had microalbuminuria lost 3.7 mL/min/year of eGFR, and those who progressed to overt proteinuria showed the highest decline in eGFR of 5.4 mL/min/year. These results show that the remission of microalbuminuria is closely related to the improvement of renal function in the long term.
Conclusion
A reduction of microalbuminuria in diabetic patients occurs more frequently than we expected. Physicians have to take care of diabetic patients with an aggressive multifactorial management plan as early as possible after the development of microalbuminuria (Table 2). The clinical target, which is important and effective for diabetic kidney disease, is to achieve the remission and/or regression of microalbuminuria. Furthermore, reducing microalbuminuria results in a risk reduction of not only renal, but also cardiovascular events.
Table 2. Option in therapy targeting the remission and regression of microalbuminuria.
| Screening of microalbuminuria and its early detection |
| Blood pressure control with the use of renin–angiotensin system blockades with hypertension and micro‐ and or macroalbuminuria; systolic blood pressure <130 mmHg |
| Good glycemic control; HbA1c <7.35% (expressed as National Glycohemoglobin Standardization Program) |
Acknowledgement
We declare no conflicts of interest.
HbA1c is expressed as National Glycohemoglobin Standardization Program.
References
- 1.Nakai S, Suzuki K, Masakane I, et al. An overview of dialysis treatment in Japan (as of December 2009) http://docs.jsdt.or.jp/overview/pdf2010/p012.pdf
- 2.Saha SA, Tuttle KR. Influence of glycemic control on the development of diabetic cardiovascular and kidney disease. Cardiol Clin 2010; 28: 497–516 [DOI] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Cravedi P, Remuzzi G, et al. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 2010; 6: 319–330 [DOI] [PubMed] [Google Scholar]
- 4.Hollenberg NK. Direct renin inhibition and the kidney. Nat Rev Nephrol 2010; 6: 49–55 [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick ES, Rigby AS, Atkin SL. The diabetes control and complications trial: the gift that keeps giving. Nat Rev Endocrinol 2009; 5: 537–545 [DOI] [PubMed] [Google Scholar]
- 6.de Zeeuw D, Agarwal R, Amdahl M. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2009; 376: 1543–1551 [DOI] [PubMed] [Google Scholar]
- 7.Staels B, Maes M, Zambon A. Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med 2008; 5: 542–553 [DOI] [PubMed] [Google Scholar]
- 8.Fioretto P, Dodson PM, Ziegler D, et al. Residual microvascular risk in diabetes: unmet needs and future directions. Nat Rev Endocrinol 2010; 6: 19–25 [DOI] [PubMed] [Google Scholar]
- 9.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414–1431 [DOI] [PubMed] [Google Scholar]
- 10.Shaw JE, Sicree RA, Zimme PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14 [DOI] [PubMed] [Google Scholar]
- 11.National Center for Chronic Disease Prevention and Health Promotion, CDC . Incidence of end‐stage renal disease attributed to diabetes among persons with diagnosed diabetes – United States and Puerto Rico, 1996–2007. MMWR Morb Mortal Wkly Rep 2010; 59: 1361–1366 [PubMed] [Google Scholar]
- 12.Yokoyama H, Kawai K, Kobayashi M, et al. Microalbuminuria is common in Japanese type 2 diabetic patients: a nationwide survey from the Japan Diabetes Clinical Data Management Study Group (JDDM 10). Diabetes Care 2007; 30: 989–992 [DOI] [PubMed] [Google Scholar]
- 13.US Renal Data System . 2010 USRDS Annual Data Report. http://www.usrds.org/reference.htm
- 14.American Diabetes Association . Nephropathy screening and treatment. Diabetes Care 2011; 34 (Suppl. 1): S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross JI, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28: 164–176 [DOI] [PubMed] [Google Scholar]
- 16.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232 [DOI] [PubMed] [Google Scholar]
- 17.Garg JP, Bakris GL. Microalbuminuria: marker of vascular dysfunction, risk factor for cardiovascular disease. Vasc Med 2002; 7: 35–43 [DOI] [PubMed] [Google Scholar]
- 18.Lane JT. Microalbuminuria as a marker of cardiovascular and renal risk in type 2 diabetes mellitus: a temporal perspective. Am J Physiol Renal Physiol 2004; 86: F442–F450 [DOI] [PubMed] [Google Scholar]
- 19.Basi S, Lewis JB. Microalbuminuria as a target to improve cardiovascular and renal outcomes. Am J Kidney Dis 2006; 47: 927–946 [DOI] [PubMed] [Google Scholar]
- 20.The Diabetes Control and Complication Trial Research Group . The absence of a glycemic threshold for the development of long‐term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996; 45: 1289–1298 [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 22.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [DOI] [PubMed] [Google Scholar]
- 23.DCCT‐EDIC . Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 2000; 342: 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin CL, Albers J, Herman WH, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006; 29: 340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holman RR, Paul SK, Bethel MA, et al. 10‐Year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589 [DOI] [PubMed] [Google Scholar]
- 26.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139 [DOI] [PubMed] [Google Scholar]
- 27.Moritz T, Duckworth W, Abraira C. Veterans Affairs diabetes trial–corrections. N Engl J Med 2009; 361: 1024–1025 [DOI] [PubMed] [Google Scholar]
- 28.The ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 29.Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail‐Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010; 340: b5444. doi: 10.1136/bmj.b5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–1418 [DOI] [PubMed] [Google Scholar]
- 33.Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370: 829–840 [DOI] [PubMed] [Google Scholar]
- 34.UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713 [PMC free article] [PubMed] [Google Scholar]
- 35.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009; 20: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004; 351: 1941–1951 [DOI] [PubMed] [Google Scholar]
- 37.Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878 [DOI] [PubMed] [Google Scholar]
- 38.Viberti GC, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure‐independent effect. Circulation 2002; 106: 672–678 [DOI] [PubMed] [Google Scholar]
- 39.Barnett AH, Bain SC, Bouter P, et al. Angiotensin‐receptor blockade versus converting–enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351: 1952–1961 [DOI] [PubMed] [Google Scholar]
- 40.Shiga Microalbuminuria Reduction Trial (SMART) Group . Reduction of microalbuminuria in patients with type 2 diabetes: the Shiga Microalbuminuria Reduction Trial (SMART). Diabetes Care 2007; 30: 1581–1583 [DOI] [PubMed] [Google Scholar]
- 41.Makino H, Haneda M, Babazono T, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 2007; 30: 1577–1578 [DOI] [PubMed] [Google Scholar]
- 42.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 43.Brenner BM, Cooper ME, de Zeeuw D, et al. for the RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 44.Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358: 2433–2446 [DOI] [PubMed] [Google Scholar]
- 45.The EUCLID Study Group . Randomised placebo‐controlled trial of lisinopril in normotensive patients with insulin‐dependent diabetes and normoalbuminuria or microalbuminuria. Lancet 1997; 349: 1787–1792 [PubMed] [Google Scholar]
- 46.Katayama S, Kikkawa R, Isogai S, et al. Effect of captopril or imidapril on the progression of diabetic nephropathy in Japanese with type 1 diabetes mellitus: a randomized controlled study (JAPAN‐IDDM). Diabetes Res Clin Pract 2002; 55: 113–121 [DOI] [PubMed] [Google Scholar]
- 47.Lewis E, Hunsicker L, Bain R, et al. The effect of angiotensin converting enzyme inhibition on diabetic nephropathy. N Engl J Med 1993; 329: 1456–1462 [DOI] [PubMed] [Google Scholar]
- 48.ONTARGET Investigators . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559 [DOI] [PubMed] [Google Scholar]
- 49.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double‐blind, controlled trial. Lancet 2008; 372: 547–553 [DOI] [PubMed] [Google Scholar]
- 50.Parving HH, Brenner BM, McMurray JJ, et al. Aliskiren trial in type 2 diabetes using cardio‐renal endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant 2009; 24: 1663–1671 [DOI] [PubMed] [Google Scholar]
- 51.Strippoli GF, Craig M, Schena FP, et al. Antihypertensive agents for primary prevention of diabetic nephropathy. J Am Soc Nephrol 2005; 16: 3081–3091 [DOI] [PubMed] [Google Scholar]
- 52.Bilous R, Chaturvedi N, Sjølie AK, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes. Three randomized trials. Ann Intern Med 2009; 151: 11–20 [DOI] [PubMed] [Google Scholar]
- 53.Mann JF, Schmeider RE, Dyal L, et al. Effects of telmisartan on renal outcomes. A randomized trial. Ann Intern Med 2009; 151: 1–10 [DOI] [PubMed] [Google Scholar]
- 54.Araki S, Haneda M, Sugimoto T, et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 2005; 54: 2983–2987 [DOI] [PubMed] [Google Scholar]
- 55.Perkins BA, Ficociello LH, Silva KH, et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348: 2285–2293 [DOI] [PubMed] [Google Scholar]
- 56.The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gæde P, Tarnow L, Vedel P, et al. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 2004; 19: 2784–2788 [DOI] [PubMed] [Google Scholar]
- 58.de Galan BE, Perkovic V, Nimomiya T, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 2009; 20: 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araki S, Haneda M, Koya D, et al. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 2007; 56: 1727–1730 [DOI] [PubMed] [Google Scholar]
- 60.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33: 278–285 [DOI] [PubMed] [Google Scholar]


