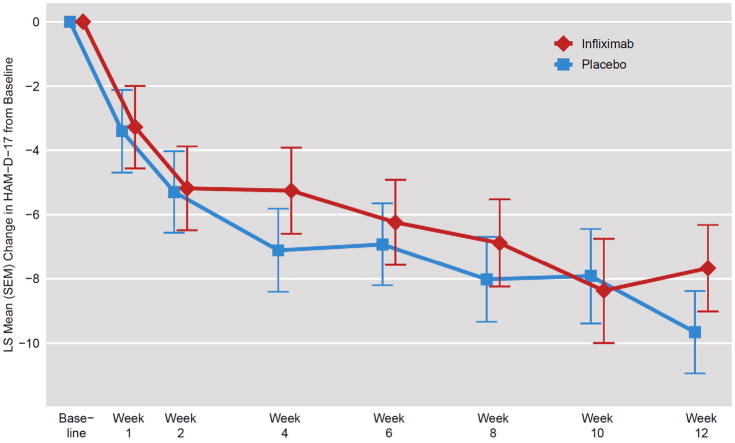
Figure 1. Change in HAM-D-17 Scores in TRD Patients Randomly Assigned to Infliximab or Placebo.
Using a double-blind, placebo-controlled, randomized design, the change in the 17-item Hamilton Depression Rating Scale (HAM-D-17) was examined in an intent-to-treat MMRM analysis of 60 patients with treatment resistant depression (TRD) administered 3 infusions of the TNF-alpha antagonist infliximab (n=30) or placebo (n=30) at baseline and 2 and 6 weeks of a 12 week trial. There was no main effect of treatment assignment or a treatment by time interaction. However, there was a significant effect of time with both groups exhibiting significant decreases in HAM-D-17 scores across treatment weeks (p=0.01). Depicted is LS Mean (SEM) Change in HAM-D-17 from Baseline to the indicated week using an unstructured covariance matrix with time as a categorical variable.