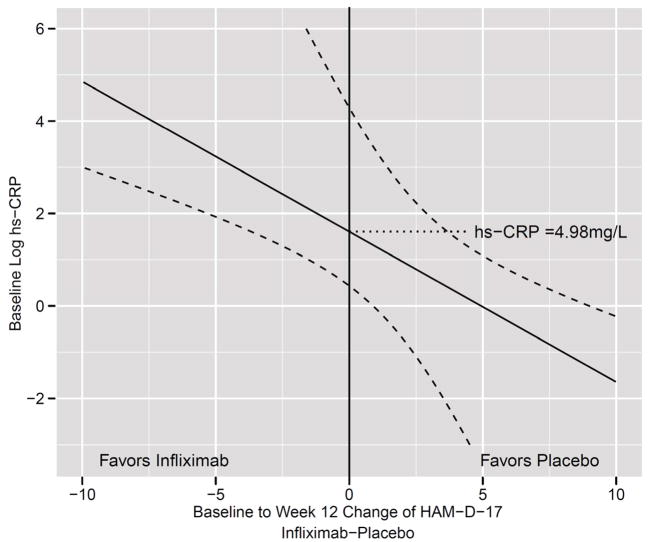
Figure 2. Relationship between Change in HAM-D-17 Score from Baseline to Week 12 (Infliximab-Placebo) and Log Baseline Plasma hs-CRP in Patients with TRD Treated with Infliximab or Placebo.
Based on an interaction between treatment, time and baseline log high sensitivity c-reactive protein (hs-CRP)(p=0.01), the relationship between baseline log plasma hs-CRP and the change in the 17-item Hamilton Depression Rating Scale (HAM-D-17) score from Baseline to Week 12 (Infliximab-Placebo) was examined in 60 patients with treatment resistant depression (TRD) administered 3 infusions of either the TNF-alpha antagonist infliximab (n=30) or placebo (n=30) at baseline and 2 and 6 weeks of a 12 week trial. At plasma concentrations of hs-CRP greater than 4.98 mg/L, the change in HAM-D-17 favored infliximab- versus placebo-treated patients, whereas at plasma concentrations below 4.98mg/L the change in HAM-D-17 favored placebo.