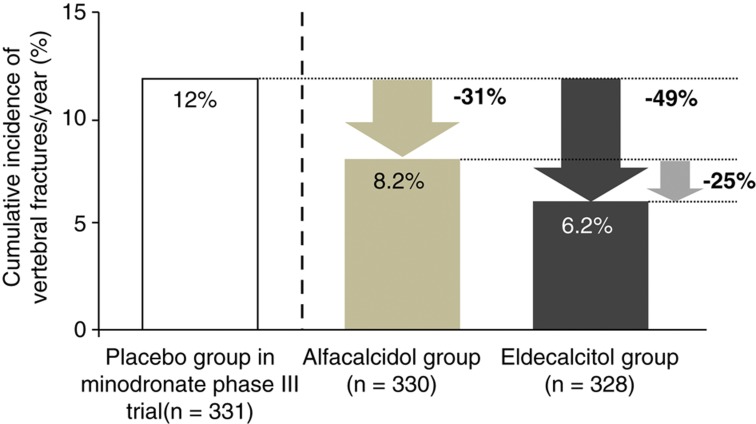
Figure 2.
Estimation of relative risk reduction of vertebral fractures compared with the placebo group of the minodronate phase III trial. Post hoc analysis was performed in the participants of fracture prevention trial with eldecalcitol who met the entry criteria of minodronate phase III trial (with one to five fragility fractures between the vertebrae Th4 and L4, and L2-4 BMD below T-score -1.7). The annual vertebral fracture incidence in alfacalcidol and eldecalcitol groups of the 3-year eldecalcitol trial was compared with that of placebo-treated patients in 2-year phase III minodronate trial. Relative risk reduction in an eldecalcitol group compared with the placebo group was estimated to be 49%, and from an alfacalcitol group was 25%, which was very similar to the 26% reduction among the whole participants of the eldecalcitol fracture prevention trial. Adapted from reference 23.