Abstract
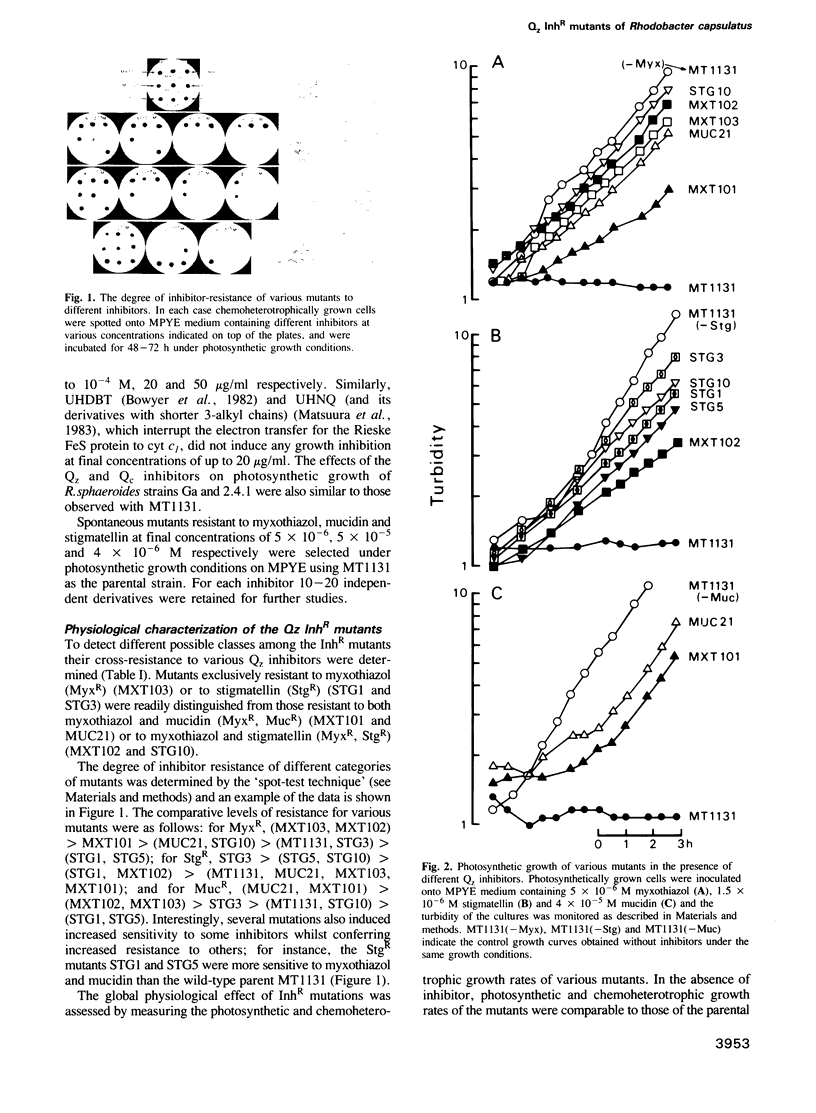
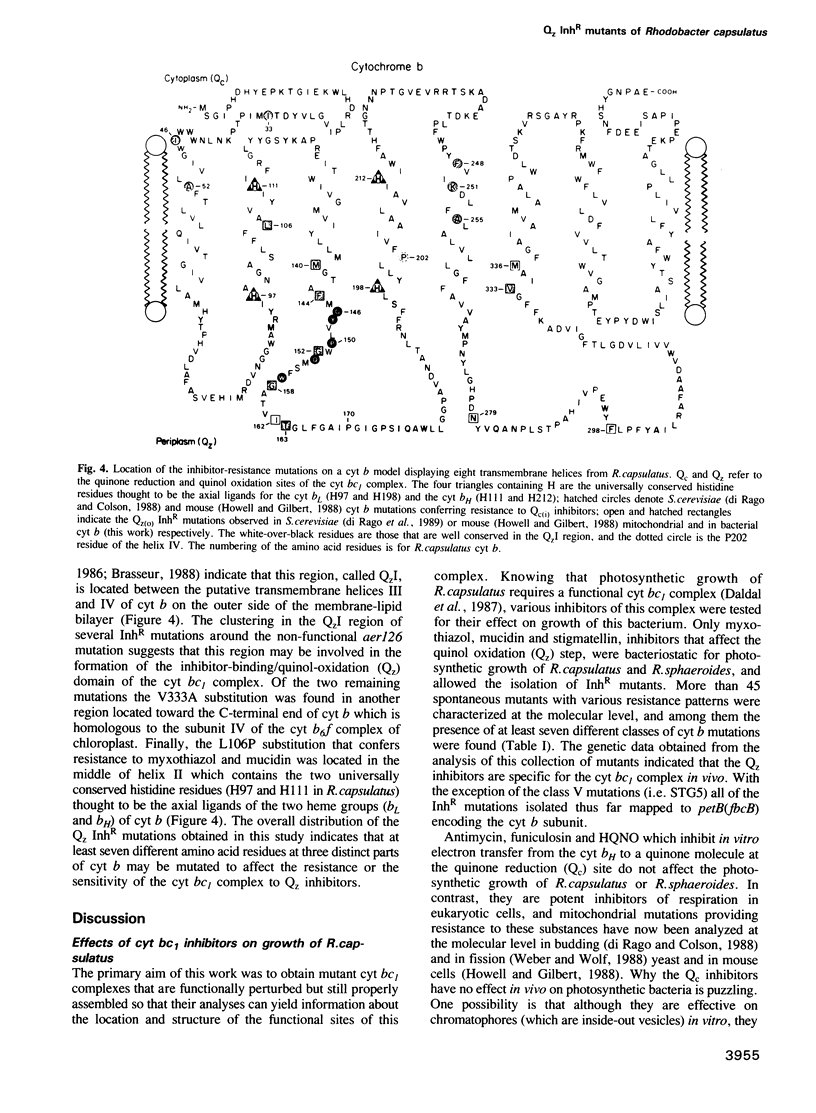
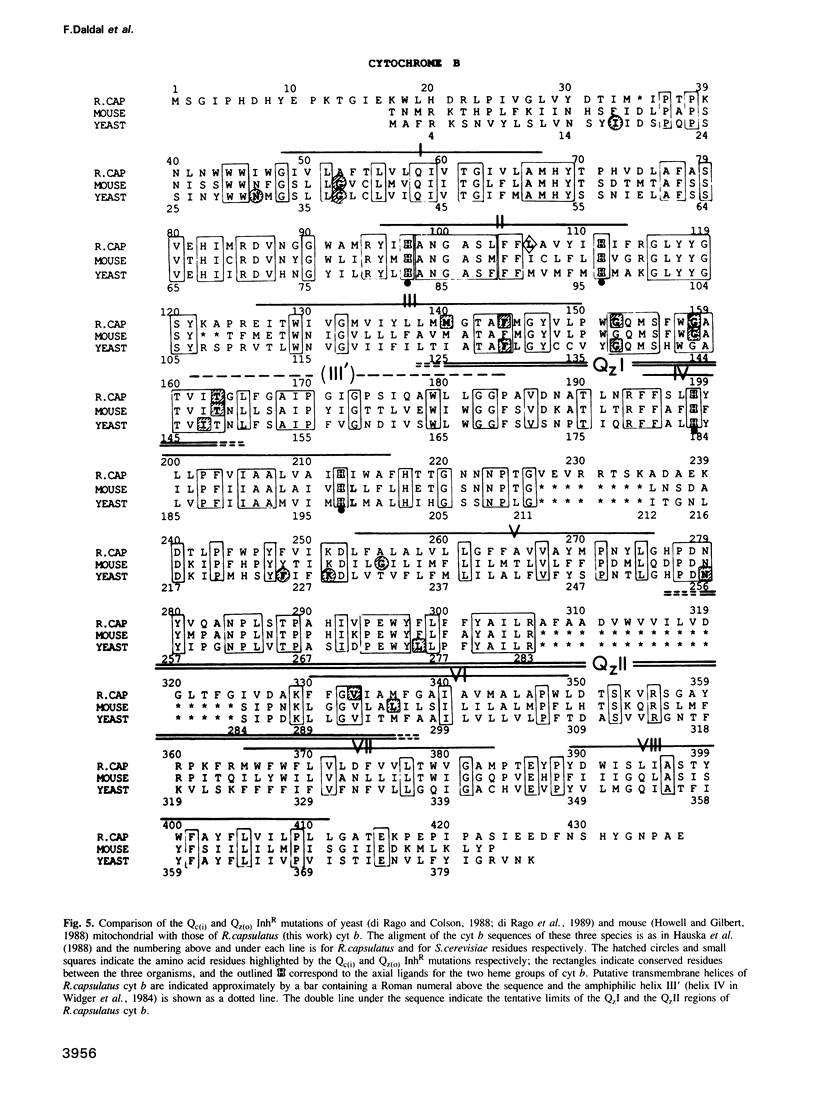
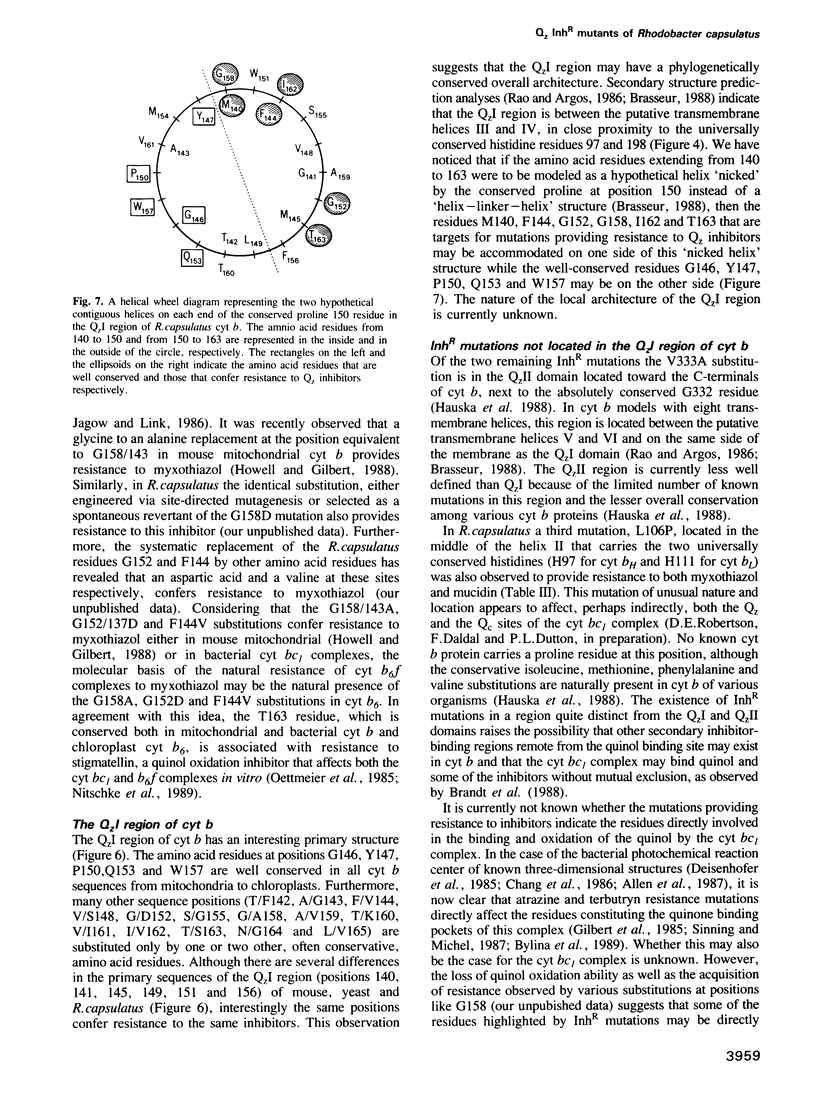
Several spontaneous mutants of the photosynthetic bacterium Rhodobacter capsulatus resistant to myxothiazol, stigmatellin and mucidin--inhibitors of the ubiquinol: cytochrome c oxidoreductase (cyt bc1 complex)--were isolated. They were grouped into eight different classes based on their genetic location, growth properties and inhibitor cross-resistance. The petABC (fbcFBC) cluster that encodes the structural genes for the Rieske FeS protein, cyt b and cyt c1 subunits of the cyt bc1 complex was cloned out of the representative isolates and the molecular basis of inhibitor-resistance was determined by DNA sequencing. These data indicated that while one group of mutations was located outside the petABC(fbcFBC) cluster, the remainder were single base pair changes in codons corresponding to phylogenetically conserved amino acid residues of cyt b. Of these substitutions, F144S conferred resistance to myxothiazol, T163A and V333A to stigmatellin, L106P and G152S to myxothiazol + mucidin and M140I and F144L to myxothiazol + stigmatellin. In addition, a mutation (aer126) which specifically impairs the quinol oxidase (Qz) activity of the cyt bc1 complex of a non-photosynthetic mutant (R126) was identified to be a glycine to an aspartic acid replacement at position 158 of cyt b. Six of these mutations were found between amino acid residues 140 and 163, in a region linking the putative third and fourth transmembrane helices of cyt b. The non-random clustering of several inhibitor-resistance mutations around the non-functional aer126 mutation suggests that this region may be involved in the formation of the Qz inhibitor binding/quinol oxidation domain(s) of the cyt bc1 complex. Of the two remaining mutations, the V333A replacement conferred resistance to stigmatellin exclusively and was located in another region toward the C terminus of cyt b. The L106P substitution, on the other hand, was situated in the transmembrane helix II that carries two conserved histidine residues (positions 97 and 111 in R. capsulatus) considered to be the axial ligands for the heme groups of cyt b. The structural and functional roles of the amino acid residues involved in the acquisition of Qz inhibitor resistance are discussed in terms of the primary structure of cyt b and in relation to the natural inhibitor-resistance of various phylogenetically related cyt bc/bf complexes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer J. R., Edwards C. A., Ohnishi T., Trumpower B. L. An analogue of ubiquinone which inhibits respiration by binding to the iron-sulfur protein of the cytochrome b-c1 segment of the mitochondrial respiratory chain. J Biol Chem. 1982 Jul 25;257(14):8321–8330. [PubMed] [Google Scholar]
- Brandt U., Schägger H., von Jagow G. Characterisation of binding of the methoxyacrylate inhibitors to mitochondrial cytochrome c reductase. Eur J Biochem. 1988 May 2;173(3):499–506. doi: 10.1111/j.1432-1033.1988.tb14026.x. [DOI] [PubMed] [Google Scholar]
- Brasseur R. Calculation of the three-dimensional structure of Saccharomyces cerevisiae cytochrome b inserted in a lipid matrix. J Biol Chem. 1988 Sep 5;263(25):12571–12575. [PubMed] [Google Scholar]
- Chang C. H., Tiede D., Tang J., Smith U., Norris J., Schiffer M. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986 Sep 1;205(1):82–86. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]
- Daldal F. Cytochrome c2-independent respiratory growth of Rhodobacter capsulatus. J Bacteriol. 1988 May;170(5):2388–2391. doi: 10.1128/jb.170.5.2388-2391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F., Davidson E., Cheng S. Isolation of the structural genes for the Rieske Fe-S protein, cytochrome b and cytochrome c1 all components of the ubiquinol: cytochrome c2 oxidoreductase complex of Rhodopseudomonas capsulata. J Mol Biol. 1987 May 5;195(1):1–12. doi: 10.1016/0022-2836(87)90322-6. [DOI] [PubMed] [Google Scholar]
- Daldal F. Molecular cloning of the gene for phosphofructokinase-2 of Escherichia coli and the nature of a mutation, pfkB1, causing a high level of the enzyme. J Mol Biol. 1983 Aug 5;168(2):285–305. doi: 10.1016/s0022-2836(83)80019-9. [DOI] [PubMed] [Google Scholar]
- Davidson E., Daldal F. Primary structure of the bc1 complex of Rhodopseudomonas capsulata. Nucleotide sequence of the pet operon encoding the Rieske cytochrome b, and cytochrome c1 apoproteins. J Mol Biol. 1987 May 5;195(1):13–24. doi: 10.1016/0022-2836(87)90323-8. [DOI] [PubMed] [Google Scholar]
- Davidson E., Daldal F. fbc operon, encoding the Rieske Fe-S protein cytochrome b, and cytochrome c1 apoproteins previously described from Rhodopseudomonas sphaeroides, is from Rhodopseudomonas capsulata. J Mol Biol. 1987 May 5;195(1):25–29. doi: 10.1016/0022-2836(87)90324-x. [DOI] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Li L. B., Yu L., Yu C. A. Identification of a Mr = 17,000 protein as the plastoquinone-binding protein in the cytochrome b6-f complex from spinach chloroplasts. J Biol Chem. 1989 Jan 25;264(3):1387–1392. [PubMed] [Google Scholar]
- Gabellini N., Harnisch U., McCarthy J. E., Hauska G., Sebald W. Cloning and expression of the fbc operon encoding the FeS protein, cytochrome b and cytochrome c1 from the Rhodopseudomonas sphaeroides b/c1 complex. EMBO J. 1985 Feb;4(2):549–553. doi: 10.1002/j.1460-2075.1985.tb03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellini N., Sebald W. Nucleotide sequence and transcription of the fbc operon from Rhodopseudomonas sphaeroides. Evaluation of the deduced amino acid sequences of the FeS protein, cytochrome b and cytochrome c1. Eur J Biochem. 1986 Feb 3;154(3):569–579. doi: 10.1111/j.1432-1033.1986.tb09437.x. [DOI] [PubMed] [Google Scholar]
- Glaser E. G., Crofts A. R. A new electrogenic step in the ubiquinol:cytochrome c2 oxidoreductase complex of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Aug 31;766(2):322–333. doi: 10.1016/0005-2728(84)90248-2. [DOI] [PubMed] [Google Scholar]
- Hauska G., Nitschke W., Herrmann R. G. Amino acid identities in the three redox center-carrying polypeptides of cytochrome bc1/b6f complexes. J Bioenerg Biomembr. 1988 Apr;20(2):211–228. doi: 10.1007/BF00768395. [DOI] [PubMed] [Google Scholar]
- Howell N., Bantel A., Huang P. Mammalian mitochondrial mutants selected for resistance to the cytochrome b inhibitors HQNO or myxothiazol. Somatic Cell Genet. 1983 Nov;9(6):721–743. doi: 10.1007/BF01539476. [DOI] [PubMed] [Google Scholar]
- Howell N., Gilbert K. Mutational analysis of the mouse mitochondrial cytochrome b gene. J Mol Biol. 1988 Oct 5;203(3):607–618. doi: 10.1016/0022-2836(88)90195-7. [DOI] [PubMed] [Google Scholar]
- Kallas T., Spiller S., Malkin R. Characterization of two operons encoding the cytochrome b6-f complex of the cyanobacterium Nostoc PCC 7906. Highly conserved sequences but different gene organization than in chloroplasts. J Biol Chem. 1988 Oct 5;263(28):14334–14342. [PubMed] [Google Scholar]
- Kallas T., Spiller S., Malkin R. Primary structure of cotranscribed genes encoding the Rieske Fe-S and cytochrome f proteins of the cyanobacterium Nostoc PCC 7906. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5794–5798. doi: 10.1073/pnas.85.16.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowski B., Ludwig B. The genes of the Paracoccus denitrificans bc1 complex. Nucleotide sequence and homologies between bacterial and mitochondrial subunits. J Biol Chem. 1987 Oct 5;262(28):13805–13811. [PubMed] [Google Scholar]
- Lang B., Burger G., Wolf K., Bandlow W., Kaudewitz F. Studies on the mechanism of electron trasport in the bc1-segment of the respiratory chain in yeast. III. Isolation and characterization of an antimycin resistant mutant ANT 8 in Schizosaccharomyces pombe. Mol Gen Genet. 1975;137(4):353–363. doi: 10.1007/BF00703260. [DOI] [PubMed] [Google Scholar]
- Ljungdahl P. O., Beckmann J. D., Trumpower B. L. Mutational analysis of the mitochondrial Rieske iron-sulfur protein of Saccharomyces cerevisiae. II. Biochemical characterization of temperature-sensitive RIP1- mutations. J Biol Chem. 1989 Mar 5;264(7):3723–3731. [PubMed] [Google Scholar]
- Ljungdahl P. O., Pennoyer J. D., Robertson D. E., Trumpower B. L. Purification of highly active cytochrome bc1 complexes from phylogenetically diverse species by a single chromatographic procedure. Biochim Biophys Acta. 1987 May 6;891(3):227–241. doi: 10.1016/0005-2728(87)90218-0. [DOI] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K., Bowyer J. R., Ohnishi T., Dutton P. L. Inhibition of electron transfer by 3-alkyl-2-hydroxy-1,4-naphthoquinones in the ubiquinol-cytochrome c oxidoreductases of Rhodopseudomonas sphaeroides and mammalian mitochondria. Interaction with a ubiquinone-binding site and the Rieske iron-sulfur cluster. J Biol Chem. 1983 Feb 10;258(3):1571–1579. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Rich P. R. A perspective on Q-cycles. J Bioenerg Biomembr. 1986 Jun;18(3):145–156. doi: 10.1007/BF00743461. [DOI] [PubMed] [Google Scholar]
- Rich P. R. Electron and proton transfers through quinones and cytochrome bc complexes. Biochim Biophys Acta. 1984 Apr 9;768(1):53–79. doi: 10.1016/0304-4173(84)90007-7. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Davidson E., Prince R. C., van den Berg W. H., Marrs B. L., Dutton P. L. Discrete catalytic sites for quinone in the ubiquinol-cytochrome c2 oxidoreductase of Rhodopseudomonas capsulata. Evidence from a mutant defective in ubiquinol oxidation. J Biol Chem. 1986 Jan 15;261(2):584–591. [PubMed] [Google Scholar]
- Robertson D. E., Dutton P. L. The nature and magnitude of the charge-separation reactions of ubiquinol cytochrome c2 oxidoreductase. Biochim Biophys Acta. 1988 Oct 5;935(3):273–291. doi: 10.1016/0005-2728(88)90223-x. [DOI] [PubMed] [Google Scholar]
- Subík J. Mucidin-resistant antimycin A-sensitive mitochondrial mutant of Saccharomyces cerevisiae. FEBS Lett. 1975 Nov 15;59(2):273–276. doi: 10.1016/0014-5793(75)80391-7. [DOI] [PubMed] [Google Scholar]
- Thöny-Meyer L., Stax D., Hennecke H. An unusual gene cluster for the cytochrome bc1 complex in Bradyrhizobium japonicum and its requirement for effective root nodule symbiosis. Cell. 1989 May 19;57(4):683–697. doi: 10.1016/0092-8674(89)90137-2. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Wu M. A., Crivellone M. Assembly of the mitochondrial membrane system. Characterization of COR1, the structural gene for the 44-kilodalton core protein of yeast coenzyme QH2-cytochrome c reductase. J Biol Chem. 1986 Dec 25;261(36):17163–17169. [PubMed] [Google Scholar]
- Von Jagow G., Gribble G. W., Trumpower B. L. Mucidin and strobilurin A are identical and inhibit electron transfer in the cytochrome bc1 complex of the mitochondrial respiratory chain at the same site as myxothiazol. Biochemistry. 1986 Feb 25;25(4):775–780. doi: 10.1021/bi00352a006. [DOI] [PubMed] [Google Scholar]
- Weber S., Wolf K. Two changes of the same nucleotide confer resistance to diuron and antimycin in the mitochondrial cytochrome b gene of Schizosaccharomyces pombe. FEBS Lett. 1988 Sep 12;237(1-2):31–34. doi: 10.1016/0014-5793(88)80165-0. [DOI] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Hu N. T., Marrs B. L. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J Mol Biol. 1979 Jun 25;131(2):157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- di Rago J. P., Colson A. M. Molecular basis for resistance to antimycin and diuron, Q-cycle inhibitors acting at the Qi site in the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J Biol Chem. 1988 Sep 5;263(25):12564–12570. [PubMed] [Google Scholar]
- di Rago J. P., Coppée J. Y., Colson A. M. Molecular basis for resistance to myxothiazol, mucidin (strobilurin A), and stigmatellin. Cytochrome b inhibitors acting at the center o of the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J Biol Chem. 1989 Aug 25;264(24):14543–14548. [PubMed] [Google Scholar]
- von Jagow G., Link T. A. Use of specific inhibitors on the mitochondrial bc1 complex. Methods Enzymol. 1986;126:253–271. doi: 10.1016/s0076-6879(86)26026-7. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Ohnishi T. The chromone inhibitor stigmatellin--binding to the ubiquinol oxidation center at the C-side of the mitochondrial membrane. FEBS Lett. 1985 Jun 17;185(2):311–315. doi: 10.1016/0014-5793(85)80929-7. [DOI] [PubMed] [Google Scholar]



