Abstract
Introduction
Testosterone (T) induces motor dysfunction in transgenic (Tg) mice overexpressing wild type androgen receptor (AR) in skeletal muscles. Since many genes implicated in motor neuron disease are expressed in skeletal muscles, mutant proteins may act in muscles to instigate muscle dysfunction in motor neuron disease.
Methods
We examined contractile properties of the extensor digitorum longus (EDL) and soleus (SOL) muscles in vitro after 5 and 3 days of T treatment in motor-impaired Tg female mice.
Results
Both muscles showed deficits in tetanic force after 5 days of T treatment, without losses in muscle mass, protein content, or fiber number. By 3 days of T treatment, only SOL showed a deficit in tetanic force comparable to that at 5 days of treatment. In both treatments, EDL shows slowed twitch kinetics, whereas SOL shows deficits in the twitch/tetanus ratio.
Conclusions
These results suggest calcium handling mechanisms in muscle fibers are defective in motor-impaired mice.
Keywords: testosterone, skeletal muscle, Kennedy’s disease, motor dysfunction, muscle dysfunction, neuromuscular disease
Introduction
The long held assumption that motor dysfunction caused by motoneuron disease is the direct result of motoneuron loss has been recently challenged. 1–2 Furthermore, whether motor impairments associated with motoneuron disease are due to defects that originate in the motoneurons, or muscles, or both has also recently come under scrutiny. Because genes linked to motoneuron disease are widely expressed, typically in both motoneurons and muscles, deficits in motor performance associated with motoneuron disease could arise from dysfunction in the motoneurons, skeletal muscles, or both. Recent data from muscle-specific mouse models of motoneuron disease suggest that skeletal muscle may indeed be a primary site of action for disease genes, and dysfunction in skeletal muscles may contribute, if not underlie, motor dysfunction.2–8
The transgenic (Tg) model used here recapitulates a polyglutamine disease called spinal bulbar muscular atrophy (SBMA),5 a late-onset motoneuron disease affecting only men and linked to a CAG repeat mutation in the AR gene. While not interfering with masculine development and function, this CAG expansion mutation in the AR gene causes men with SBMA to show a progressive loss of muscle strength that greatly impairs their motor function.9 This muscle-specific or “myogenic” Tg model was engineered by inserting a transgene into mice that overexpresses wild type (WT) rat androgen receptors (AR) selectively in skeletal muscle fibers using the human skeletal α actin promoter.5 Like SBMA in humans and other mouse models of this disease, motor dysfunction in such myogenic Tg mice is triggered by male levels of androgens and can be relieved by lowering circulating androgen levels.5,10–15 While Tg females in this model also express the AR transgene at comparably high levels, they are asymptomatic throughout life. However, when provided with male-typical levels of testosterone, motor function of Tg females rapidly deteriorates, developing motor defects in only a few days comparable to those seen in Tg males, including shortened stride length and deficits in grip strength.5,16 Significantly, Tg male mice with a mutation in the endogenous AR gene, which eliminates endogenous AR function, also show the same androgen-dependent loss of motor function,17 demonstrating that testosterone activates functional transgenic AR to impair motor function. While Tg males show several markers of motoneuron disease, including atrophic and angulated muscle fibers, and deficits in the number of axons in ventral roots, acutely diseased Tg females do not.5,16 Thus, AR acting directly in muscles can produce profound deficits in motor function without the expected histopathology in muscle, and/or loss of motoneurons, a dissociation that is also evident in other mouse models of SBMA.12–13,15
To explore whether muscle dysfunction might underlie motor dysfunction in myogenic mice, we directly examined the contractile properties of muscles from diseased and healthy female mice. We chose to study females over males, because unlike Tg males, who are chronically impaired, the disease state can be “turned on” by exposure to androgens in Tg females. Thus, given that the time of disease onset is known, acutely-impaired Tg females offer the potential of sorting out proximate pathogenic mechanisms underlying motor dysfunction from secondary effects of disease. Muscle mechanics of the fast-twitch extensor digitorum longus (EDL) and slow-twitch soleus (SOL) were examined in vitro from adult Tg females and age-matched WT controls following 5 and 3 days of testosterone treatment. We observed a rapid loss of intrinsic force in both the EDL and SOL that occurs independent of muscle mass in this model.
Methods
Animals and hormone treatment
Transgenic (Tg) mice were generated and genotyped using PCR as previously described.5 Tg animals from the 141 founding line express a WT rat AR cDNA under the control of the human skeletal α actin promoter. Tg females were mated to C57/B6J mice for several generations to produce progeny for this study. Water and food were provided ad libitum. All animal procedures were approved and performed in compliance with the Michigan State University Institutional Animal Care and Use Committee, in accordance with the standards in the NIH Guide for the Care and Use of Laboratory Animals. Adult (90–120 days) Tg and age-matched wild type (WT) females were deeply anesthetized with isoflurane and Silastic capsules (1.57 mm i.d. and 3.18 mm o.d., effective release length of 6 mm) containing either crystalline testosterone (T) (~0.15 mg per capsule) or nothing (blank) were implanted subcutaneously just caudal to the scapula as previously described.16 Incisions were closed with 9 mm surgical staples. All animals received the analgesic ketoprofen (5 mg/kg, sc) immediately following surgery. In the first experiment, mice were treated for 5 days before muscle function was examined. Treatment groups were as follows: blank-treated wild type (B WT), testosterone-treated wildtype (T WT), blank-treated transgenic (B Tg), and testosterone-treated transgenic (T Tg) with n=5–6 mice/group. Separate cohorts of mice were used to examine the EDL and the SOL after 5 days of treatment. In a second experiment, contractile properties of the EDL and SOL harvested from the same set of mice were examined after 3 days of T treatment, comparing muscles from T WT to T Tg mice, n=5–6 mice/group.
Motor function tests
Motor function was assessed using the grip strength and hang tests one hour before surgery to establish baseline performance (Day 0) and on each day following surgery (Days 1–3 or 1–5) up to the time of sacrifice. Forelimb grip strength was assessed using a grip strength meter (Columbus Instruments) oriented in the horizontal plane.16,15,12 Mice were held by their tails and lowered to the apparatus, allowed to grasp the metal grid with their forelimbs only, and were pulled by their tail vertically at a 90° angle away from the grid until their grip was released. Force (g) applied to the bar at the moment of release was recorded as grip strength. Seven consecutive measurements were done within a single two minute session for each subject with the lowest and highest grip values excluded, and the mean of the remaining five values recorded as the grip strength for that animal and session. The hang test was conducted by placing mice on a wire grid that was then turned upside-down 40 cm above a counter. Latency to fall (up to 120 seconds) from the wire grid was measured.5,18
In vitro studies to assess muscle mechanics
To determine whether impairments in skeletal muscle function might underlie motor dysfunction, muscle contractile characteristics of the EDL and SOL were examined in vitro in motor-impaired Tg females and controls after 3 or 5 days of hormone treatment. These hindlimb muscles were selected because the EDL and SOL are the prototypical fast and slow twitch muscles, respectively, with considerable background information available on their normal contractile properties.19–20 Moreover, we were interested in determining whether the toxic effects of an activated AR in this model system might depend on fiber type, as observed in other diseases.21–22
Mice were deeply anesthetized with a single intraperitoneal injection of sodium pentobarbital in saline solution (0.1 mg/g body weight, Abbott Laboratories, North Chicago, IL, USA). We chose sodium pentobarbital as opposed to ketamine/xylazine as our anesthetic since it has been shown to have little to no residual effect on the contractile properties of muscle when assessed in vitro, yielding estimates that best fit those reported in the literature.23 Once the muscles were exposed, 5-0 silk suture was tied to the proximal and distal tendons in situ, and muscle resting length was recorded. Muscles were then excised by cutting above the suture knot on the proximal tendon and below the suture knot distally. Excised muscles were incubated in an 8 mL organ bath containing oxygenated Ringer’s (117.1 mM NaCl, 4.6 mM KCl, 25.3 mM NaHCO3, 2.5 mM CaCl, 1.2 mM MgSO4; pH 7.4; equilibrated with 95% O2/5% CO2).24–25 Temperature of the bath was continuously monitored using a K-type thermocouple (Omega Engineering, Stamford, CT) adjacent to the muscle and maintained at 25°C±0.2°C by circulating water through a glass-jacketed organ bath (Radnoti Glass Technology, Monrovia, CA). Isolated muscles were fixed at their approximate resting length by tying one end of the muscle ligature to a glass hook and the other to an isometric force transducer (Astro-Med, West Warwick, RI) fitted with a positioning micrometer. Muscles were initially set to their in vivo resting length by using the suture distances between the proximal and distal tendons and then the optimal length (Lo) for each muscle was precisely determined using the length-tension relation. Electrical stimulation was delivered via two platinum plate electrodes (3–4 mm apart, 25 mm long) parallel to the long axis of the muscle using a Grass S88 Stimulator (Grass Instruments, Quincy, MA). Force output was digitized using an analog-to-digital converter (ADC) (model AT MIO16E; National Instruments, Austin, TX) controlled via commercially available software (LabScribe/NI; iWorx, Dover, NH) operating at a sampling rate of 1 KHz (1 msec time resolution). Muscles were stimulated to produce successive twitches (1 ms duration, 1 Hz) at 10–60 volts (V) to determine the supramaximal stimulation voltage (voltage at which maximal twitch force was elicited X 1.35) used subsequently during the experiment (generally 30–40 V). Although the voltage used to directly stimulate the SOL muscle after five days of treatment was slightly higher for diseased mice (T Tg) compared to the other groups, there was statistically no difference in stimulation voltages between treatments and genotypes for the SOL and EDL muscles at either the five or three day time point. After muscle excision, mice were euthanized.
Muscles function was assessed based on directly evoked twitches and tetani. Stimulation parameters used to evoke muscle twitches were 1 ms duration pulses delivered at rates ranging from 0.2 Hz to 120Hz at supramaximal voltage to generate the full range of function from single twitch to fused tetani. This data was used to generate the force-frequency relation for each preparation. Muscle tetani were evoked by stimulating the muscle directly with 1 ms duration pulses at fusion frequency, with fusion frequency operationally defined as the frequency at which ripples in the plateau phase of the tetanic force are ≤ 3% of the peak force 24. The fusion frequency ranged from 80–100 Hz for the EDL and 30–40 Hz for the SOL. Because the kinetics of force development are slower in the SOL muscles, the length of the stimulus train to induce the plateau phase of a tetanus also differed between muscle fiber types. Tetanic contractions were elicited by delivering a 500 ms train to EDL and 1 s train to the SOL. In all instances both the twitch and tetanic peak forces were normalized to the tendon-free muscle mass. Resistance to fatigue was assessed by stimulating the EDL and SOL with repeated tetani stimuli (1 train/s; 330 ms duration, ~80–100 Hz in EDL and 670 ms duration, 30–40 Hz in SOL) until force decreased to approximately 10% of initial force. This was experimentally determined as 90 s for wild type EDL and 135 s for wild type SOL from start of the fatigue stimulation, and this parameter was used for all treatment groups in this study. Fatigue resistance was measured by determining the duration in seconds it took for maximal tetanic force to drop to half its starting value. Following the experiment, muscles were rapidly removed from the apparatus, dissected free from tendons, blotted of excess media, and weighed to estimate mass and then stored at −80°C.
In a separate series of experiments with other mice (B WT mice and T Tg, 90–120 days old; n=2–4/group), D-tubocurarine was added to bath (100 μM, Sigma-Aldrich) to test whether the motor nerve contributed to the force produced.26 Force production was not affected by the addition of curare, indicating that field applied electrodes were not relying on stimulation of neuromuscular junctions to elicit muscle contractions (data not shown).
Histology
In a separate cohort of age-matched WT and Tg females (90 –120 days of age) treated for 5 days with either T or blank capsules, the SOL was dissected, placed in OCT compound (Sakura Japan) in cryomolds (Cryomolds) and snap frozen in liquid nitrogen (n = 5 mice/group). SOL were sectioned on a Leica cryostat at 10 μm, thaw-mounted onto gelatin-coated slides, and stained with hematoxylin and eosin (H&E) for counting fibers, as previously described for the EDL.16
Androgen receptor protein expression
Upon completion of force recordings, we assessed total protein content and AR expression in muscles using previously described methods.27 In brief, the EDL and SOL were homogenized in lysis buffer (14 mM NaCl, 0.268 mM KCl, 0.147 mM KH2PO4, 0.802 mM Na2HPO4 1% Nonidet P-40 (v/v), 0.5% sodium deoxycholate (wt/v), 1% sodium dodecyl sulfate (wt/v), 10% protease inhibitor (v/v; Sigma-Aldrich P2714)) using a Bio-Gen PRO 200 homogenizer (PRO Scientific). Samples were centrifuged at 12,000x at room temperature for five minutes. Supernatant from each sample was isolated to determine protein concentration using a Pierce BCA protein assay (Thermo Scientific) and a spectrophotometer (Beckman DU 530). Ten μg of protein was loaded per lane on an 8% Tris-Glycine gel (Invitrogen), run at 125 V for two hours, transferred to a nitrocellulose membrane at 45 V for two hours and probed using a rabbit polyclonal antiserum directed at AR (N-20, Santa Cruz Biotechnology; Santa Cruz, CA, USA; dilution 1:500; 0.4 μg/mL) followed by incubation of the membrane in anti-rabbit goat antiserum labeled with horseradish peroxidase (HRP; Santa Cruz, sc-2004; 0.4 μg/mL) and detected by Luminol (Santa Cruz Biotechnology). Films were exposed and developed using an X-OMAT 1000A Processor (Kodak). After probing for AR, nitrocellulose membranes were stripped and re-probed for actin using goat polyclonal anti-actin antiserum directly conjugated to HRP (I-19 Santa Cruz; 0.4 μg/mL).
Protein gels
Muscle homogenates were prepared as previously described and loaded into 12% SDS gels. Gels were run until the dye front had reached the bottom of the gel (~two hours at 125 V). Protein bands were visualized by incubating in Coomassie Brilliant Blue R250 (Thermo Scientific Pierce) and destaining (40% methanol, 10% acetic acid v/v in distilled water).
Testosterone levels
Blood was collected via a transcardial puncture from deeply anesthetized mice used for studies on muscle mechanics, and held on ice for 30 minutes before centrifuging at 3000 rpm for 20 minutes at 8° C (Sorvall Biofuge Fresco, Thermo Scientific, Asheville, NC). After centrifugation of blood samples, plasma was collected and held at −80° C until assayed for circulating testosterone levels as previously described.28
Data analysis and statistics
Analysis of mechanical transients was performed using a custom algorithm29 in a MatLab programming environment (Mathworks, Natick, MA). In brief, raw digital signals are passed through a low-pass filter to smooth the data and then analyzed for force output and tension-time integrals. Kinetic parameters for the rise time (time to peak force) and relaxation time (time of return to baseline) were also measured in individual muscle twitches as previously described.29 Statistical analysis of data after 5 days of treatment was performed using two-way analysis of variance with genotype and hormone treatment as independent variables (SPSS Inc., Chicago, IL). Group differences were considered significant at p<0.05 and post hoc comparisons were done using the Tukey post-hoc test. Independent groups T-tests were used to evaluate significant differences between muscles from Tg versus WT mice after 3 days of T treatment.
Results
Testosterone (T) treatment impairs motor function without loss of muscle mass
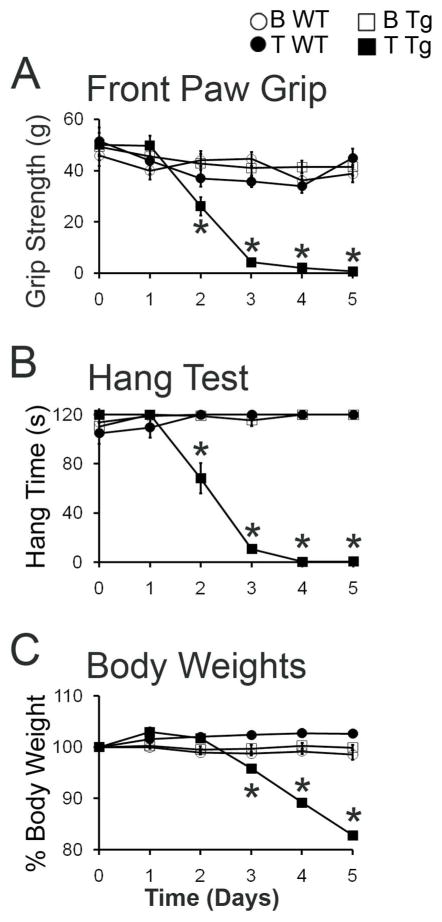
At Day 0, before testosterone treatment, comparisons across treatment groups indicated similar body weights (data not shown) and comparable motor function based on both the hang and grip strength tests (Fig 1A, B). Replicating previous findings,5,16 T induced a rapid and progressive decline in motor performance only in Tg mice. None of the three control groups (B WT, T WT, B Tg) showed any change in motor function. Although T also induced decreases in the body weight of Tg females (Figure 1C), losses in body weight lagged significantly behind the loss of muscle strength, as seen previously.16 For example, a significant deficit in hang time was detected by day two of T treatment in Tg females (p < 0.001, compared to T WT or B Tg), with performance on this test reaching 0 s by Day 3, whereas a significant loss in body weight did not emerge until 3 days of T treatment (p < 0.05 at Days 3–5 in T Tg females versus asymptomatic controls T WT or B Tg). These data indicate that a rapid loss of motor function occurs only when T activates transgenic AR in skeletal muscle fibers and that neither the AR transgene nor T alone affect motor performance, replicating previous results.5,16
Figure 1.
Motor function declines rapidly in testosterone (T)-treated adult female transgenic (Tg) mice that express a wild-type androgen receptor (AR) transgene only in skeletal muscle fibers. Baseline performance at Day 0 before hormone treatment did not differ across treatment groups. T treatment induced a rapid decline in grip strength (A), hang time (B), and body weight (C) only in Tg females. By Day 5, motor performance on both tests were significantly decreased for T-treated Tg (T Tg) females compared to baseline or compared to the other groups, including asymptomatic females that were blank-treated wild-type (B WT), testosterone-treated wild-type (T WT), and blank-treated transgenic (B Tg). Each asymptomatic group maintained pre-treatment performance levels on both the grip strength and hang tests across the 5 days of treatment indicating that only the combination of AR transgene expression in muscle fibers and male levels of androgens instigates motor dysfunction. Values are means ± standard errors of means based on N = 5–6 mice/muscle and group. *p≤0.05, T Tg versus B Tg or T WT.
Although T induced marked losses in both body weight and muscle strength based on the hang and grip strength tests in Tg females, 5 days of T treatment did not induce any apparent decrease in muscle mass. Specifically, neither the EDL nor SOL muscle of Tg females showed a significant decrease in mass following T treatment (Table 1).
Table 1.
Biometric data
| Treatment Groups | |||||
|---|---|---|---|---|---|
| Wild Type | Transgenic | ||||
| B | T | B | T | ||
| Muscle Weights | EDL | 8.0±0.4 | 8.6±0.2 | 9.5±0.3 | 8.6±0.3 |
|
| |||||
| (mg) | SOL | 7.6±0.2 | 8.3±0.6 | 9.3±0.4 | 9.4±0.4 |
|
| |||||
| Plasma T Levels | 1.4±0.3 | 29.3±1.8 | 1.2±0.1 | 49.5±5.9 | |
| (nmol/L) | |||||
|
| |||||
| Normalized Peak Twitch Force | EDL | 6.67±0.73 | 7.48±0.42 | 7.20±0.47 | 4.21±0.40* |
| (N/g) | SOL | 3.22±0.20 | 3.38±0.23 | 3.25±0.19 | 0.45±0.04* |
|
| |||||
| Twitch/Tetanus Ratio | EDL | 0.21±0.01 | 0.24±0.01 | 0.21±0.01 | 0.24±0.01 |
| (5 Day T) | SOL | 0.17±0.01 | 0.18±0.01 | 0.21±0.00 | 0.12±0.01* |
| EDL | NA | 0.27±0.02 | NA | 0.23±0.02 | |
| (3 Day T) | SOL | NA | 0.21±0.02 | NA | 0.13±0.02* |
|
| |||||
| Protein Content (mg)/Muscle weight (g) | EDL | 47.6±8.8 | 66.0±14.8 | 53.1±7.4 | 41.5±6.6 |
| SOL | 52.2±18.5 | 66.9±12.1 | 43.5±13.2 | 55.9±10.1 | |
T, testosterone; mg, milligrams; nmol/L, nanomolar per liter; N/g, newtons/gram. Values are means ± standard error of means.
p<0.05.
As expected, circulating T levels were greater in females given T capsules (T WT, T Tg) than those given blanks (B WT, B Tg; Table 1, p<0.05). However, T capsules of the same size and randomly selected from the same stock produced greater levels of circulating T titers in Tg females than WT females, but both averages were within the physiological range of gonadally intact WT male mice. We suspect that this elevated level of T in Tg mice is due to their reduced body weight by 5 days of T treatment, as we have obtained similar findings in rats that differ in body weight.30
Skeletal muscles from T-treated Tg female mice show striking androgen-dependent loss of force
Five-Day Treatment
Tetanic Force
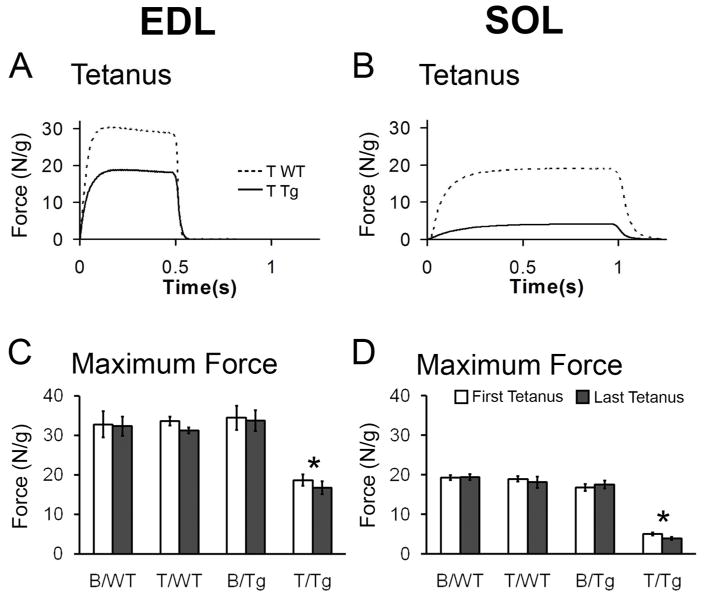
Tetanic force measurements from WT or Tg mice with normal motor function indicate that the EDL and SOL exhibit the expected characteristics of fast and slow muscle, respectively, with the fast-twitch EDL requiring less time but higher stimulation frequencies than the slow-twitch SOL to attain maximal force production (data not shown). Also as expected, the EDL produces nearly twice as much force per gram of muscle wet weight as the SOL (Fig 2A-D). While these same fiber-type specific differences were also seen in the EDL and SOL muscles of motor-impaired, T-treated mice, both muscles showed striking deficits in maximal tetanic force normalized to muscle mass (Fig 2). T induced a significant decrease in maximal tetanic force only in Tg females, resulting in a significant interaction of genotype with treatment and significant main effects of both genotype and treatment (ps < 0.001). Both the EDL and SOL muscles of T-treated Tg females showed significantly less tetanic force compared to either T WT or B Tg females (ps < 0.001). T also induced a greater proportional deficit in tetanic force in the SOL than in the EDL of motor-impaired mice. While tetanic force for the fast-twitch EDL from T-treated Tg mice was approximately half that produced by the EDL from control mice, tetanic force for the slow-twitch SOL was decreased even further in T-treated Tg mice, to about a quarter of that produced by controls. All muscles tested had comparable force production at the end of the experiment as at the beginning (Fig 2C, D), indicating that there was little deterioration of the muscles in vitro, and suggesting that the force deficits observed in vitro reflect primary muscle dysfunction in vivo.
Figure 2.
Tetanic force produced by both EDL and SOL of motor-impaired female mice is significantly reduced compared to tetanic force produced by corresponding muscles of asymptomatic controls after five days of testosterone (T) treatment. Representative traces for T WT muscles and T-treated transgenic (T Tg) muscles are shown (A, B). Tetanic force produced by the EDL of T Tgs is about half of control females (C) whereas tetanic force produced by the SOL of T Tg females is about a quarter of T WT controls (D). Tetanic force produced by muscles of control-treated Tg females (B/Tg) is equivalent to that of WTs, indicating that AR transgene expression per se does not impair muscle strength. Comparable force deficits are observed at the beginning and end of experiments (indicated as first and last tetanus) for both EDL and SOL of T Tg mice, suggesting force deficit is present in vivo and is not due to a more rapid deterioration of these muscles in vitro. Force (in Newtons-N) was normalized to muscle wet weight (grams-g). Values in histogram are means ± standard errors of means, N = 5–6 mice/group. *p<0.001, T Tg versus B Tg or T WT.
Muscle histology and protein content
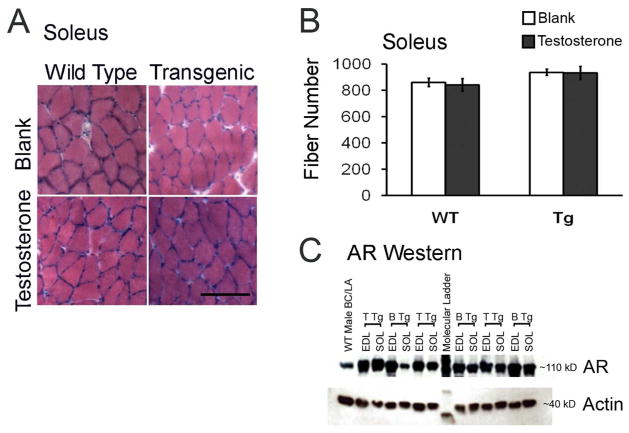
The SOL was chosen for detailed histological analyses because we had previously established that the EDL from motor-impaired Tg mice treated with T for 9 days shows no notable histopathology in H&E stained cross sections.16 Likewise, we found no signs of histopathology in the SOL of motor-impaired, T-treated Tg mice. For example, muscle fibers in the SOL from T-treated Tg mice appear homogeneous in size (Fig 3A), without evidence of atrophy. While centralized nuclei were occasionally observed, they were no more frequent in the impaired SOL than in control SOL (data not shown). Moreover, we found no differences in the number of SOL muscle fibers from motor-impaired versus control mice (Fig 3B).
Figure 3.
Neither histopathology nor expression of the AR transgene explains the severe deficit in SOL force production of T-treated Tg mice. Cross sections of SOL muscle (scale bar = 500 μm) from Tg females after 5 days of T treatment stained with hematoxylin and eosin exhibit no signs of abnormal morphology (e.g. fiber atrophy/hypertrophy, centralized nuclei, A) nor alterations in fiber number (B) compared to the SOL from other treatment groups (N = 4–5 mice/group). Bars represent means ± standard errors of means. AR immunoblot (C) showing AR expression levels in the EDL and SOL from asymptomatic (B Tg) versus symptomatic (T Tg) mice. We find no consistent differences in AR expression (~110 kD) between EDL and SOL muscles, indicating that a difference in AR protein content between the EDL and SOL is not likely to underlie the relatively greater force deficit in the SOL compared to the EDL in T-treated Tg mice. Actin (~40 kD) loading control shows no consistent differences in protein loading between the EDL and SOL of Tg mice.
Because loss of contractile proteins might not be reflected in overall muscle weight, we also measured the total amount of protein in the EDL and SOL muscles of motor-impaired and control mice but we found no evidence for a net loss in total protein in either the EDL or SOL of motor-impaired Tg mice compared to control Tg and WT mice with normal motor function (Table 1). Moreover, muscle protein gels revealed no consistent differences in specific contractile proteins in either muscle (data not shown).
We also addressed whether the SOL expresses AR at a higher level than the EDL in Tg females, since this could potentially explain the more severe loss of force in the SOL than the EDL following T-treatment (Fig 2). To assess this possibility, AR expression was examined in Western blots of muscle homogenates from Tg mice. As expected, AR expression was higher in both the EDL and SOL of Tg mice compared to the AR-enriched bulbocavernosus/levator ani (BC/LA) muscles of WT mice,5 but there were no consistent differences in AR expression between the two muscles (Fig 3C) that might account for the relatively greater loss of tetanic force induced by T in the SOL compared to the EDL from Tg mice (Fig 2).
Skeletal muscles from T-treated Tg females exhibit androgen-dependent changes in twitch force and kinetics
Twitch Force
Peak force of individual twitches (normalized to muscle weight) produced by the EDL and SOL from T-treated Tg females were decreased to about 60% and 7%, respectively, of peak twitch forces produced by these same muscles from blank-treated Tg mice (Table 1; Fig 4A, B). Although the twitch to tetanus ratio did not differ across treatment groups in the EDL (Table 1; p > 0.05), SOL from T-treated Tg females had a significantly lower twitch to tetanus ratio than muscles from Tg and WT control mice (Table 1; p < 0.001), due to T treatment reducing SOL peak twitch force more than peak tetanic force in Tg mice.
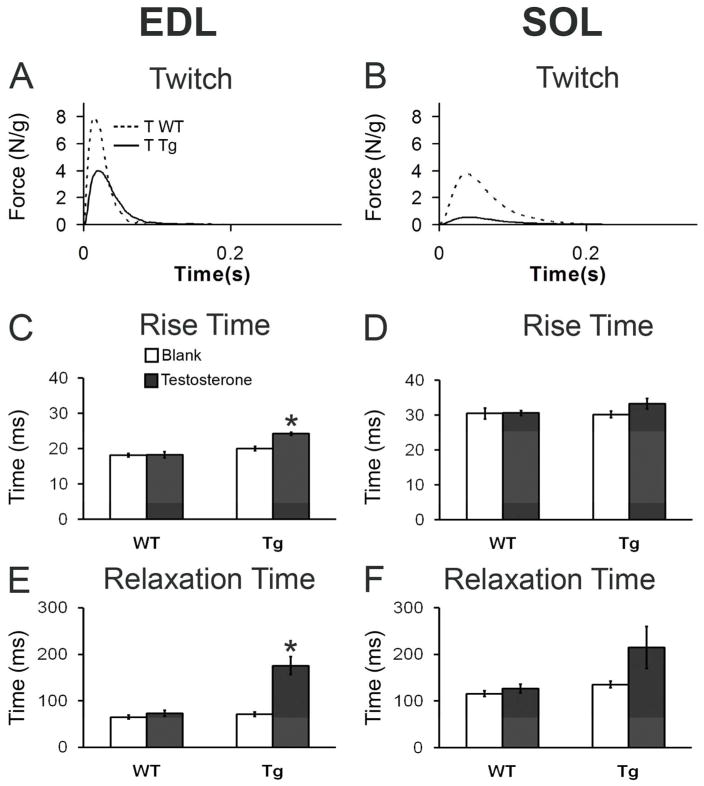
Figure 4.
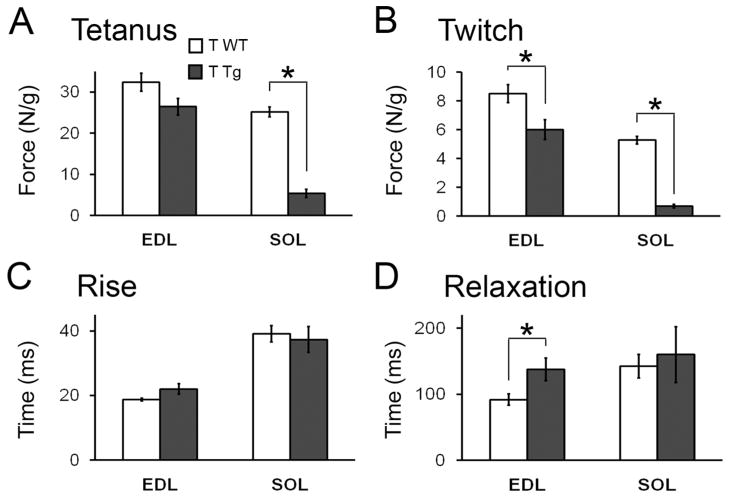
Twitch kinetics are slowed only in the EDL of motor-impaired Tg mice after 5 days of T treatment. Representative twitches from the fast-twitch EDL and the slow-twitch SOL (A, B) reveal decreased peak twitch force for both muscles of T-treated Tg mice (see Table 1 for mean values) but altered twitch kinetics only for the EDL. Quantitative analysis of individual twitches (C – F) confirms a significant prolongation in the time it takes to reach peak twitch force (C) and to relax (E) in EDL but that neither parameter is significantly altered by T in the SOL muscle of Tg mice (D, F). Plotted values are means ± standard errors of means, with N = 5–6 mice/group. *p<0.001 T Tg versus B Tg or T WT.
Contraction Kinetics
Analysis of twitch kinetics revealed a slower rise time to peak force and a slower relaxation time to baseline in EDL from motor-impaired T-treated Tg mice compared to the EDL from control-treated Tg or WT mice (Fig 4C, E; ps < 0.001 for both rise and relaxation in T Tg versus T WT or B Tg). Delays in both the rise and relaxation times suggest the kinetics of calcium release from, and uptake to, the sarcoplasmic reticulum or binding to troponin C of the thin filament are altered in the EDL of T-treated Tg mice. The observed left-ward shift in the force-frequency curves in T-treated EDL also supports this view (data not shown). However, neither the times to peak force nor relaxation time of the twitch were significantly altered by T in the SOL of Tg mice (Fig 4D, F).
Tetanic force production is reduced in the SOL but not EDL of Tg mice after three days of T treatment
Deficits in motor function are observed as early as 2 days after the initiation of treatment, with T-treated Tg mice unable to perform the hang test by Day 3, raising the question of whether changes in muscle function are also evident this early. Thus, we measured muscle mechanics in a separate cohort of Tg and WT females given T for 3 days.
We found the same time course of motor dysfunction after 3 days of T treatment as seen in the previous cohort (data not shown). In vitro measures of contractile properties after 3 days of T treatment revealed that both peak tetanic and twitch forces were significantly reduced in the slow-twitch SOL of Tg mice compared to the SOL of WT mice (p < 0.05, Fig 5A, B) but that only peak twitch force was significantly decreased in the EDL from the same Tg mice at this timepoint (p < 0.05, Fig 5B). Maximal tetanic force produced by the EDL in Tg mice was slightly but not significantly less than in WT controls (p=0.07, Fig 5A). Only the SOL muscle of Tg mice showed a significant deficit in the twitch-to-tetanus ratio after 3 days of T exposure (p < 0.01, Table 1), comparable to the effects of T after 5 days of treatment. Twitch kinetics for the SOL of Tg mice were unaffected by T after 3 days of treatment, as expected. However, for the EDL of Tg mice, in which both speed to contract and relax were prolonged after 5 days of T, only relaxation was significantly slowed after 3 days of T treatment (p < 0.05, Fig 5D), with no effect of T on rise time to peak force (Fig 5C). In sum, the same pattern of deficits were seen in the SOL after both 3 and 5 days of T treatment whereas only some deficits, namely peak twitch force and relaxation time, were seen in the EDL after both 5 and 3 days of T treatment, suggesting that deficits in the SOL generally emerge sooner than in the EDL.
Figure 5.
The SOL but not the EDL of motor-impaired mice shows the same deficit in peak tetanic force after 3 days of T treatment. Tetanic force in the slow-twitch SOL from T Tg mice was significantly decreased after 3 days of T treatment (A), comparable to the magnitude of the effect of 5 days of treatment (Fig 2). A subtle, but not significant difference was seen in EDL from the same motor-impaired mice (T Tg versus T WT mice, p = 0.07). However, peak twitch force in both muscles was significantly reduced in T Tg mice compared to T WT mice (B). Twitch kinetics were largely unaffected in T-treated Tg mice after 3 days of treatment (C, D) except that relaxation time was significantly slowed in EDL, consonant with the effect of T on this parameter after 5 days of treatment (Fig 4). N = 5–7/group. *p<0.05.
Only the EDL from motor-impaired mice show deficits in fatigue resistance
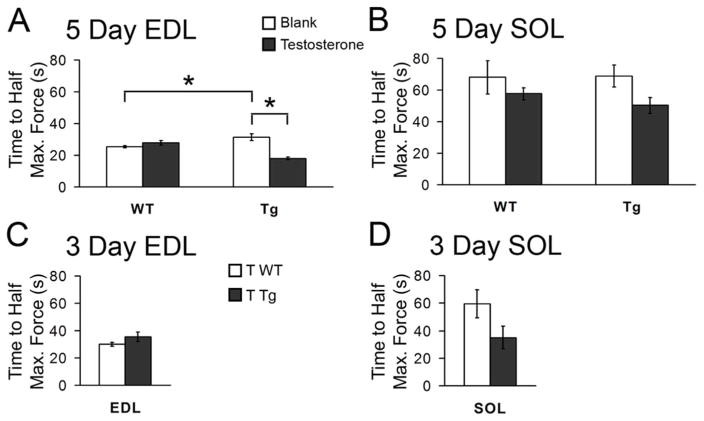
The only effects on fatigue resistance were seen on the EDL after 5 days of T treatment. The EDL from Tg mice treated with T for 5 days fatigued significantly faster, dropping to half maximal tetanic force sooner than the EDL from either blank-treated Tgs or T-treated WTs (Fig 6A, p < 0.01). Interestingly, the EDL from blank-treated Tg mice however showed a greater resistance to fatigue than the EDL from blank-treated WT mice (p < 0.05), indicating that the transgene alone enhanced fatigue resistance in the EDL (Fig 6A). We found no effect of T on fatigue resistance in the SOL from motor-impaired Tg mice after either 3 or 5 days of T treatment (Fig 6B, D) nor did we find an effect of T on fatigue resistances in the EDL of Tg mice after 3 days of treatment (Fig 6D). That deficits in both maximal tetanic force and fatigue resistance in the EDL emerge later than other defects in this muscle suggests that the same mechanism, perhaps involving mitochondrial dysfunction, may underlie both.
Figure 6.
The EDL but not the SOL from motor-impaired Tg mice showed compromised resistance to fatigue after 5, but not 3, days of T treatment. Fatigue resistance was measured as time in seconds from onset of tetanizing stimulation to when the muscles decreased in force by 50% of maximal tetanic force. The EDL from Tg mice after 5 days of T treatment fatigued significantly faster, dropping to half maximal force several seconds sooner than the EDL from either T-treated WT or blank-treated Tg mice (A). Interestingly, the EDL from blank-treated Tg mice showed a greater resistance to fatigue than the EDL from blank-treated WT (B Tg versus B WT), indicating a beneficial effect of the transgene itself on resistance to fatigue in the EDL. Five days of T treatment had no effect on fatigue resistance in SOL (B) nor did we see any effect of T on fatigue resistance after 3 days of treatment in either muscle from Tg mice (C and D). 5-day EDL: N = 5–6 mice/group; 5-day SOL, N = 5–6 mice/group; 3-day EDL, N = 6 mice/group; 3-day SOL, N = 4–7 mice/group. *p≤0.05.
Discussion
The current study shows that male levels of androgens not only trigger a pronounced and rapid loss of motor function in female Tg mice, but also trigger a concomitant loss of contractile function in the skeletal muscles of such mice, with maximal force production of both the fast twitch EDL and slow twitch SOL plummeting to about half that of WT controls. This marked loss in contractile strength develops within a few days, in step with the emergence of motor dysfunction, and occurs without detectable losses in muscle mass or protein. Given that the AR transgene is expressed only in skeletal muscle fibers in these Tg mice,5 our data show that androgens can act directly on muscle fibers to reduce their contractile strength. Because ARs are normally expressed in skeletal muscle,27,31–32 mutant AR may act directly in muscle, independent of the motoneurons, to cause muscle weakness in SBMA. Importantly, the deleterious effects of transgenic AR on both motor and muscle function were evident only when Tg mice are exposed to male-like levels of androgens, in line with the androgen-dependence of SBMA in humans.
We have confirmed that the loss in force production reflects neither a loss in muscle mass nor a disproportionate decay in muscle function in vitro. While force production of muscles from all four treatment groups declined somewhat during the course of the experiment, this decline was no greater in muscles from T-treated Tg mice than in muscles from control mice (Fig 2), indicating that the reduced force exhibited by the EDL and SOL muscles of motor-impaired mice cannot be attributed to a more rapid demise of muscle function in vitro. Moreover, while muscle mass generally correlates with the amount of force produced, the robust loss of maximal tetanic force to less than half that of normal was not accompanied by reduced muscle mass in motor-impaired mice. This was surprising, particularly because the mice lose nearly 20% of their body weight during the five day treatment period. This weight loss likely reflects the increased metabolic rate and loss of fat stores triggered by androgen activation of transgenic AR in muscle which is known to occur in such myogenic rodent models.33 Indeed, at the end of the five day treatment, fat pads in T-treated Tg females are negligible in size (unpublished observation). While measures of twitch and tetanic force were normalized to the wet weight of each muscle, which takes into account any change in mass that might contribute to changes in force, we also saw no change in muscle mass per se, based either on their weight or total protein content (Table 1). Neither did we see any apparent histological changes. The impaired SOL contained a normal number of fibers, and those fibers appeared intact, with no signs of atrophy ordinarily associated with deficits in muscle strength (Fig 3). Our results in the SOL also agree well with prior results from the EDL of similarly treated mice.16 Given that myosin and actin make up most of the bulk in muscle fibers, these data suggest that AR perturbs the contractile capacity of skeletal muscles in motor-impaired mice not by inducing protein loss but by perturbing the function of critical proteins mediating muscle contraction either directly or through modulating calcium handling properties.
Contractile strength was more severely and more rapidly affected in the SOL than the EDL in T-treated Tgs, losing about 75% of its force after only 3 days of androgen exposure, a time when effects on the EDL are negligible (Fig 5). This finding raised the question of whether transgenic AR is expressed at appreciably higher levels in the SOL than in the EDL. However, AR Westerns revealed no consistent difference in AR content between the EDL and SOL of Tg mice. It is also noteworthy that the EDL of Tg mice showed other androgen-dependent defects in contractile properties that the Tg SOL did not. These results also indicate that differences in AR expression can not readily explain the more severe effects of T on force in the SOL.
While deficits in contractile force favored the SOL, the kinetics of force development showed the opposite pattern, with only the EDL of Tg mice affected by T, showing a significant slowing in both the rise and relaxation times compared to control-treated mice (Fig 4). We also found that fatigue resistance was reduced in the EDL, but not in the SOL, after 5 days of T treatment in Tg mice, with no effects on fatiguability at 3 days (Fig 6). Interestingly, other investigators studying the same model report that the number and size of mitochondria in the EDL increases significantly after a week of T exposure, 34 suggestive of mitochondrial dysfunction. While the EDL normally derives much of its ATP from glycolytic metabolism, the EDL from female Tgs after 9 days of T has shifted to a more oxidative metabolism based on NADH staining.16 This apparent shift toward oxidative metabolism in the face of possibly impaired mitochondrial function could explain why fatigue resistance is reduced in the EDL after 5 days of T. In sum, these findings suggest that there are other inherent differences between these muscles, perhaps related to their fast- versus slow-twitch fiber type composition,19 that predispose them to respond differentially to the toxic effects of an activated AR.
The overall profile of defects in both the EDL and SOL of motor-impaired mice suggest that mechanisms controlling intracellular calcium fluxes in muscle fibers may be perturbed. For example, the prolonged rise time to peak twitch force in the EDL of T-treated Tg mice suggests that the function of the ryanodine receptor (RyR1) may be impaired. This receptor controls the release of intracellular calcium from the sarcoplasmic reticulum to trigger muscle contraction. Likewise, the decreased twitch to tetanus ratio seen in SOL after both 3 and 5 days of T treatment, as well as the loss of tetanic force, suggests inefficient or impaired release of calcium from the sarcoplasmic reticulum controlled by the RyR1. Moreover, the prolonged relaxation times seen at both 3 and 5 days of T treatment in the EDL of Tg mice suggest that the sarco(endo)plasmic reticulum calcium ATPases (SERCA), which pumps calcium back up into the sarcoplasmic reticulum to allow muscles to relax, may also be impaired.35
While it is possible that other mechanisms involved in the initiation of muscle contraction may be impaired,, such as troponin C which binds calcium to trigger contraction, compelling evidence suggests that post-translational modifications to the RyR1 may have a role in the contractile defects we find. Recent work examining the mechanisms behind a loss of contractile strength in both aging and dystrophic muscles indicates that the RyR1 becomes modified by reactive oxygen and nitrogen species, causing the receptor to become leaky, and leading to a deficit in muscle force.36–38 Moreover, mitochondria likely play a key role in these maladaptive changes to the RyR1. In our model, loss of muscle function is associated with both an increase in mitochondrial number and activity of complexes I, II and IV in the respiratory chain of mitochondria.34 These mitochondrial complexes are the primary source of reactive nitrogen and oxygen. Paradoxically, the production of ATP may at the same time be decreasing, as suggested by the reduced resistance to fatigue in the EDL and a deficit in citric synthase activity found in the fast twitch muscle anterior tibialis of these same T-treated Tg mice (unpublished observation). Together, these data are consistent with the idea that excessive amounts of reactive oxygen and nitrogen species produced by dysfunctional mitochondria in muscles of T-treated Tg mice may modify the RyR1, impairing its function. Such modifications to the RyR1 could explain many, if not all, the defects in muscle contraction that we observe. It is also noteworthy that such changes in the RyR1 can occur quite rapidly. A bout of intense exercise has been shown to induce such changes in the RyR1 in less than 24 hours,36 consistent with the rapid decline in muscle force that we observe, particularly in the slow twitch SOL.
The current data from our Tg mouse model may be relevant to human disease, particularly SBMA, an androgen-dependent motoneuron disease that affects only men9,14,39–40 Several different mouse models of SBMA have been developed that broadly express a full length human AR harboring the disease-causing CAG repeat expansion. Such SBMA models show androgen-dependent loss of motor function.15,12–13 However, because the mutated AR is expressed in both skeletal muscle and motoneuron in these various models, whether mutant AR acts in one or both of these cell types or in some other cell population is unknown. While androgen-dependent motor dysfunction in the current model is triggered by WT AR acting exclusively in muscle fibers, such mice show a disease phenotype remarkably similar to that of other SBMA mouse models, indicating that AR may act in muscle to directly cause muscle weakness in SBMA. Consistent with this view, humans with SBMA show elevated levels of serum creatine kinase (CK), indicative of skeletal muscle damage.41 Such elevations are observed in patients with primary myopathies, such as muscular dystrophy but not in patients that have primary neuropathies.42 Evidence from a knock-in (KI) mouse model of SBMA also suggests that primary myopathy is caused by muscle AR.12 Significantly, muscles from KI males show signs of pathology before the motoneurons. Affected muscles in those males also have decreased expression of the CLCN1 chloride channel, and show abnormal spontaneous activity, like muscles affected by myotonic dystrophy, a primary myopathy. Finally, given that WT AR and the polyglutamine-expanded AR can exert toxicity through common mechanisms,43 it is possible that SBMA pathogenesis originates through different pathways of which mutant AR is only one such route, not unlike other neurodegenerative diseases where known genetic mutations account for only a minority of the cases. Recognizing that an apparently normal AR protein can cause disease by a failure of mechanisms which ordinarily limit its inherent toxic potential could explain why some individuals diagnosed with SBMA due to muscle weakness and reduced androgen sensitivity, nonetheless lack the CAG expansion in their AR gene.44 In short, SBMA could be caused by different pathogenic events that perturb normal AR function in comparable ways, triggering the same disease. Because the AR transgene is expressed exclusively in muscle fibers of our myogenic model but nonetheless leads to expression of an SBMA phenotype, these findings are consistent with the possibility that AR acts directly in muscle fibers to cause or substantively contribute to SBMA pathogenesis.
Acknowledgments
Funding for this project was provided by R01 NS045195 (CLJ) and DK095210 (RWW). The authors would like to thank the following individuals for their contributions to this work. Animals used in the experiments were bred and generated with the assistance of Diane Redenius. Data analysis using MatLab was done with the assistance of Matt Latourette and Chris Hunley. Technical assistance for the histology was provided by Kelsey Korabik. Citrate synthase information was collected by Ken Less.
Abbreviations
- AR
androgen receptor
- B
blank
- EDL
extensor digitorum longus
- SBMA
spinal and bulbar muscular atrophy
- SOL
soleus
- T
testosterone
- Tg
transgenic
- WT
wild type
References
- 1.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26(34):8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, Halter B, Huze C, Schaeffer L, Bouillaud F, Loeffler JP. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS One. 2009;4(4):e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun S, Croizat B, Lagrange MC, Warter JM, Poindron P. Constitutive muscular abnormalities in culture in spinal muscular atrophy. Lancet. 1995;345(8951):694–695. doi: 10.1016/s0140-6736(95)90869-2. [DOI] [PubMed] [Google Scholar]
- 4.Cifuentes-Diaz C, Frugier T, Tiziano FD, Lacene E, Roblot N, Joshi V, Moreau MH, Melki J. Deletion of murine SMN exon 7 directed to skeletal muscle leads to severe muscular dystrophy. J Cell Biol. 2001;152(5):1107–1114. doi: 10.1083/jcb.152.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monks DA, Johansen JA, Mo K, Rao P, Eagleson B, Yu Z, Lieberman AP, Breedlove SM, Jordan CL. Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proc Natl Acad Sci U S A. 2007;104(46):18259–18264. doi: 10.1073/pnas.0705501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10(5):608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19(11):2284–2302. doi: 10.1093/hmg/ddq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischbeck KH. Kennedy disease. J Inherit Metab Dis. 1997;20(2):152–158. doi: 10.1023/a:1005344403603. [DOI] [PubMed] [Google Scholar]
- 10.Banno H, Katsuno M, Suzuki K, Takeuchi Y, Kawashima M, Suga N, Takamori M, Ito M, Nakamura T, Matsuo K, Yamada S, Oki Y, Adachi H, Minamiyama M, Waza M, Atsuta N, Watanabe H, Fujimoto Y, Nakashima T, Tanaka F, Doyu M, Sobue G. Phase 2 trial of leuprorelin in patients with spinal and bulbar muscular atrophy. Ann Neurol. 2009;65(2):140–150. doi: 10.1002/ana.21540. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, Chang C. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13(3):348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 12.Yu Z, Dadgar N, Albertelli M, Gruis K, Jordan C, Robins DM, Lieberman AP. Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J Clin Invest. 2006;116(10):2663–2672. doi: 10.1172/JCI28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, Sang C, Kobayashi Y, Doyu M, Sobue G. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35(5):843–854. doi: 10.1016/s0896-6273(02)00834-6. [DOI] [PubMed] [Google Scholar]
- 14.Kinirons P, Rouleau GA. Administration of testosterone results in reversible deterioration in Kennedy’s disease. J Neurol Neurosurg Psychiatry. 2008;79(1):106–107. doi: 10.1136/jnnp.2006.101899. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier-Larsen ES, O’Brien CJ, Wang H, Jenkins SC, Holder L, Lieberman AP, Merry DE. Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci. 2004;24(20):4778–4786. doi: 10.1523/JNEUROSCI.0808-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen JA, Yu Z, Mo K, Monks DA, Lieberman AP, Breedlove SM, Jordan CL. Recovery of function in a myogenic mouse model of spinal bulbar muscular atrophy. Neurobiol Dis. 2009;34(1):113–120. doi: 10.1016/j.nbd.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen JA, Troxell-Smith SM, Yu Z, Mo K, Monks DA, Lieberman AP, Breedlove SM, Jordan CL. Prenatal flutamide enhances survival in a myogenic mouse model of spinal bulbar muscular atrophy. Neurodegener Dis. 2011;8(1–2):25–34. doi: 10.1159/000313682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sopher BL, Thomas PS, Jr, LaFevre-Bernt MA, Holm IE, Wilke SA, Ware CB, Jin LW, Libby RT, Ellerby LM, La Spada AR. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 2004;41(5):687–699. doi: 10.1016/s0896-6273(04)00082-0. [DOI] [PubMed] [Google Scholar]
- 19.Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushmerick MJ, Moerland TS, Wiseman RW. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci U S A. 1992;89(16):7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkin JD, Scott RL, West JM, Lopes E, Quah AK, Cheema SS. Properties of slow- and fast-twitch muscle fibres in a mouse model of amyotrophic lateral sclerosis. Neuromuscul Disord. 2005;15(5):377–388. doi: 10.1016/j.nmd.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol. 2008;586(14):3337–3351. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapointe BM, Cote CH. Anesthetics can alter subsequent in vitro assessment of contractility in slow and fast skeletal muscles of rat. Am J Physiol. 1999;277(3 Pt 2):R917–921. doi: 10.1152/ajpregu.1999.277.3.R917. [DOI] [PubMed] [Google Scholar]
- 24.Wiseman RW, Beck TW, Chase PB. Effect of intracellular pH on force development depends on temperature in intact skeletal muscle from mouse. Am J Physiol. 1996;271(3 Pt 1):C878–886. doi: 10.1152/ajpcell.1996.271.3.C878. [DOI] [PubMed] [Google Scholar]
- 25.Dentel JN, Blanchard SG, Ankrapp DP, McCabe LR, Wiseman RW. Inhibition of cross-bridge formation has no effect on contraction-associated phosphorylation of p38 MAPK in mouse skeletal muscle. Am J Physiol Cell Physiol. 2005;288(4):C824–830. doi: 10.1152/ajpcell.00500.2004. [DOI] [PubMed] [Google Scholar]
- 26.Cairns SP, Chin ER, Renaud JM. Stimulation pulse characteristics and electrode configuration determine site of excitation in isolated mammalian skeletal muscle: implications for fatigue. J Appl Physiol. 2007;103(1):359–368. doi: 10.1152/japplphysiol.01267.2006. [DOI] [PubMed] [Google Scholar]
- 27.Johansen JA, Breedlove SM, Jordan CL. Androgen receptor expression in the levator ani muscle of male mice. J Neuroendocrinol. 2007;19(10):823–826. doi: 10.1111/j.1365-2826.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 28.Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Jayaraman RC, Latourette MT, Siebert JE, Wiseman RW. A rapid algorithm for processing digital physiologic signals: Application to skeletal muscle contractions. Biomedical Signal Processing and Control. 2006;1:307–313. [Google Scholar]
- 30.Johnson RT, Schneider A, DonCarlos LL, Breedlove SM, Jordan CL. Astrocytes in the rat medial amygdala are responsive to adult androgens. J Comp Neurol. 2012;520(11):2531–2544. doi: 10.1002/cne.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monks DA, O’Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473(1):59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- 32.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89(10):5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 33.Fernando SM, Rao P, Niel L, Chatterjee D, Stagljar M, Monks DA. Myocyte Androgen Receptors Increase Metabolic Rate and Improve Body Composition by Reducing Fat Mass. Endocrinology. 2010 doi: 10.1210/en.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musa M, Fernando SM, Chatterjee D, Monks DA. Subcellular effects of myocyte-specific androgen receptor overexpression in mice. J Endocrinol. 2011;210(1):93–104. doi: 10.1530/JOE-11-0071. [DOI] [PubMed] [Google Scholar]
- 35.Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80(3):1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 36.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105(6):2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15(3):325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14(2):196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt BJ, Greenberg CR, Allingham-Hawkins DJ, Spriggs EL. Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology. 2002;59(5):770–772. doi: 10.1212/wnl.59.5.770. [DOI] [PubMed] [Google Scholar]
- 41.Chahin N, Sorenson EJ. Serum creatine kinase levels in spinobulbar muscular atrophy and amyotrophic lateral sclerosis. Muscle Nerve. 2009;40(1):126–129. doi: 10.1002/mus.21310. [DOI] [PubMed] [Google Scholar]
- 42.Pearce JM, Pennington RJ, Walton JN. Serum Enzyme Studies in Muscle Disease. Ii. Serum Creatine Kinase Activity in Muscular Dystrophy and in Other Myopathic and Neuropathic Disorders. J Neurol Neurosurg Psychiatry. 1964;27:96–99. doi: 10.1136/jnnp.27.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nedelsky NB, Pennuto M, Smith RB, Palazzolo I, Moore J, Nie Z, Neale G, Taylor JP. Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron. 2010;67(6):936–952. doi: 10.1016/j.neuron.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mariotti C, Castellotti B, Pareyson D, Testa D, Eoli M, Antozzi C, Silani V, Marconi R, Tezzon F, Siciliano G, Marchini C, Gellera C, Donato SD. Phenotypic manifestations associated with CAG-repeat expansion in the androgen receptor gene in male patients and heterozygous females: a clinical and molecular study of 30 families. Neuromuscul Disord. 2000;10(6):391–397. doi: 10.1016/s0960-8966(99)00132-7. [DOI] [PubMed] [Google Scholar]