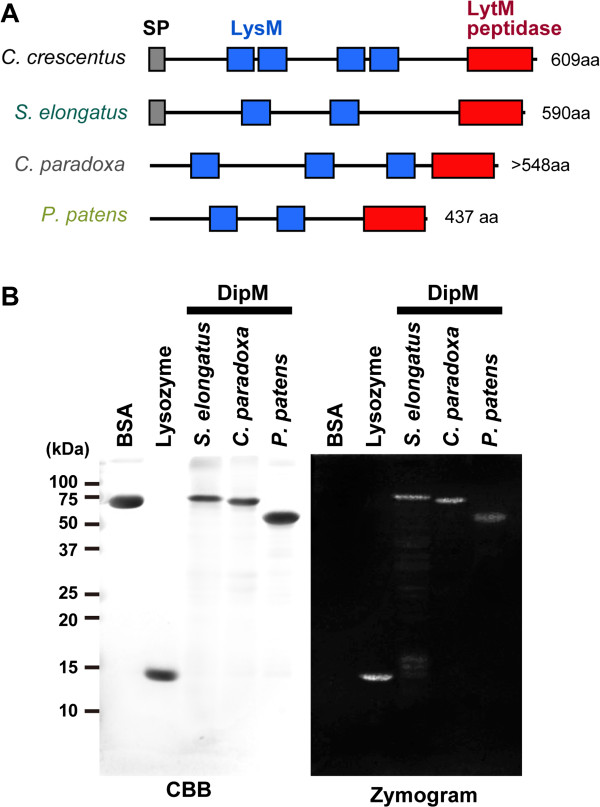
Figure 2.
DipM homologs of cyanobacteria and photosynthetic eukaryotes display PG hydrolase activity. (A) Predicted domain structure of C. crescentus DipM and its homologs in the cyanobacterium S. elongatus, the glaucophyte alga C. paradoxa and the moss P. patens. SP, signal peptide. The GenBank accession numbers are summarized in Additional file 1: Table S1. For the P. patens DipM proteins, only DipM1 is shown. For C. paradoxa DipM, the deduced amino acid sequence lacks information on the N-terminal portion because we were unable to obtain full length cDNA. (B) Zymogram analysis of the PG hydrolase activity of the DipM homologs. S. elongatus (cyanobacterium), C. paradoxa (glaucophyte) and P. patens (moss) DipM homologs hydrolyzed murein sacculi in the gel (zymogram) which is indicated by negative staining with methylene blue. 5 μg of bovine serum albumin (BSA), lysozyme and recombinant DipM polypeptides were applied to SDS gels containing purified S. elongatus murein sacculi. The proteins in one of the gels were stained with Coomassie Brilliant Blue (CBB). The other gel was incubated in renaturation buffer and then areas of lysis were detected by staining of the murein sacculi with methylene blue. For the P. patens DipM proteins, only DipM1 was examined.