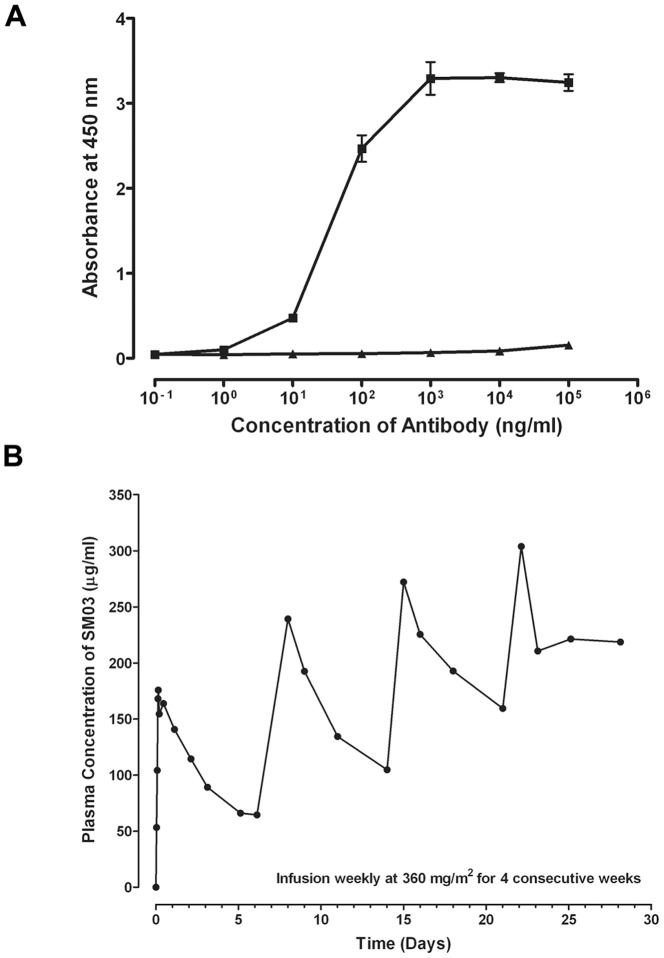
Figure 7. Pharmacokinetic measurement of chimeric anti-CD22 SM03 by capture ELISA.
The anti-Id scFv Hc5 was coated onto 96-well ELISA plate at 10 µg/mL, and then incubated at 37°C for 1 h with (A) various amounts of chimeric anti-CD22 SM03 (▪) or control chimeric anti-TNFα N009 (▴) to establish a standard calibration curve; (B) blood samples of a lymphoma patient treated with weekly infusion of 360 mg/m2 of chimeric anti-CD22 SM03 to determine residual SM03 in plasma as described in Methods. The captured SM03 antibodies were then detected by incubating with secondary HRP-conjugated goat anti-human IgG Fc antibody. Data shown is a representative showing the typical pharmacodynamic measurement of circulating SM03 in patients undertook the Phase I clinical trial.