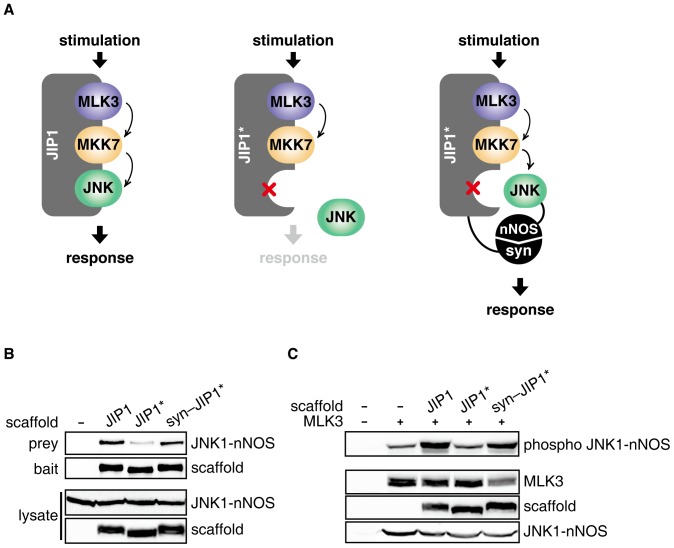
Figure 1. JNK MAP kinase pathway was restored by replacing JIP1-JNK docking interaction with PDZ interaction domains.
A. The wiring strategy of the JNK MAP kinase pathway is illustrated. The diagram shows a conventional JIP1-dependent JNK MAP kinase pathway (left). A JIP1* scaffold containing mutations to disrupt docking interaction with JNK leads to release of JNK from the scaffold complex and, therefore, a decrease in the signaling response (middle). The functional scaffold complex is reassembled by re-recruiting missing JNK to the scaffold complex using fusions of syn-JIP1* and JNK1-nNOS, resulting in the restoration of JNK signaling (right). To recruit JNK to JIP1*, syn and nNOS PDZ domains were fused to JIP1* and JNK, respectively. B. JIP1, JIP1* or syn-JIP1* scaffold was co-expressed with Flag-JNK1-nNOS in 293T cells. Myc-tagged scaffold proteins were immunoprecipitated using an anti-myc antibody conjugated to agarose. The bound Flag-JNK1-nNOS was detected by immunoblotting. C. To verify that the alternatively assembled scaffold complex restored the signaling response, dual-phosphorylation of JNK1-nNOS in the whole cell lysates was detected by western blot analysis using an anti-phospho JNK antibody. Expression of HA-MLK3 was used to stimulate the JIP1-mediated JNK pathway in 293T cells [13]. Each experiment described here was performed in triplicate.