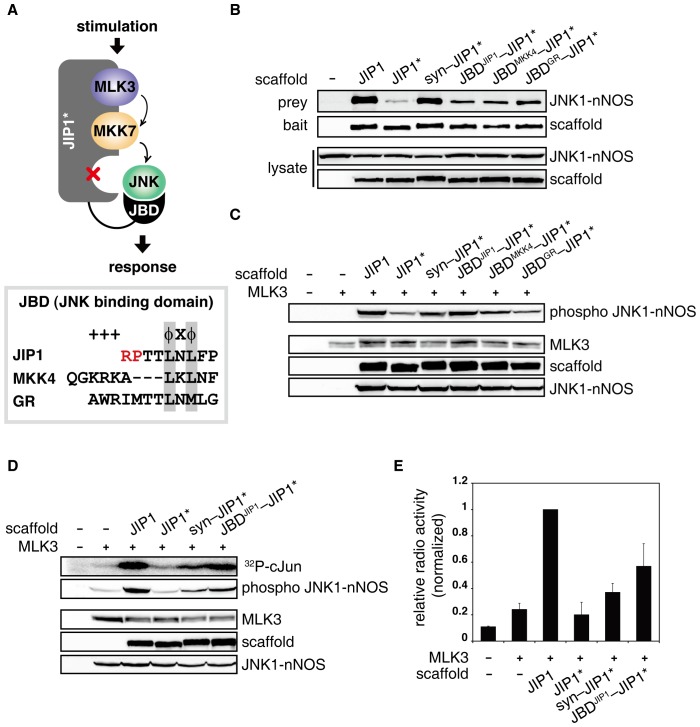
Figure 2. JNK MAP kinase pathway was restored by re-recruiting JNK using JBDs.
A. The diagram illustrates the reassembly of JIP1 scaffold complex by re-recruiting the missing JNK using JBDs. Peptides encoding JBDs were fused to the N-terminus of JIP1* to recruit JNK to the scaffold complex. The box shows the alignment of the amino acid sequences of the following JBDs used in this experiment: mouse JIP1 (156–164), human MKK4 (38–48), and mouse GR (571–582). Basic (+) and hydrophobic (Φ) residues in the consensus sequence were indicated. Conserved hydrophobic residues among the sequences were boxed in gray. Arginine and proline residues mutated in JIP1* were indicated in red. B. Flag-JNK1-nNOS was co-expressed with JIP1 variants (JIP1, JIP1*, syn-JIP1*, JBDJIP1-JIP1*, JBDMKK4-JIP1* or JBDGR-JIP1*) in 293T cells. Interactions between JNK1-nNOS and JIP1 variants were analyzed as described in Figure 1B. C. Dual phosphorylation of Flag-JNK1-nNOS in the whole cell lysates was analyzed as described above to monitor the JNK signaling restored by reassembly of JIP1 complex via JBDs. Expression of HA-MLK3 was used to stimulate JIP1-mediated JNK pathway in 293T cells [13]. D and E. JNK signaling mediated by JIP1 scaffold reassembly was monitored by an in-gel kinase assay to measure the catalytic activity of activated JNK in the whole cell lysates. cJun protein was used as a substrate for JNK. Expressed proteins and phosphorylated JNK were examined by immunoblotting. Samples were separated in a gel containing GST-cJun, and catalytic activity of JNK was quantified by measuring the radioactivity incorporated in GST-cJun using a liquid scintillation counter. Each experiment was performed in triplicate and repeated at least three times.