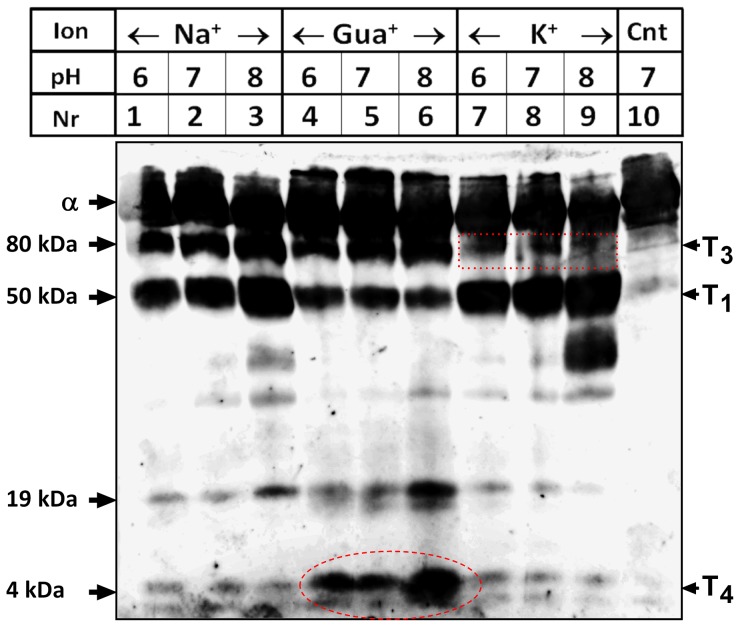
Figure 7. Trypsin cleavage in the presence of substrate ions.
Trypsin cleavage of pig kidney Na+,K+-ATPase was performed in the presence of Tris buffer and 50 mM of NaCl, Gua+ hydrochloride, or KCl. The enzyme (100 µg) was allowed to equilibrate with ions for 30 min at 20°C, following by the addition of trypsin (trypsin/protein ratio ∼1∶100). In controls (Cnt), water replaced trypsin. The reaction was allowed to proceed for 40 min and was terminated using SDS sample buffer acidified with TCA. Protein fragments were resolved using SDS-PAGE and visualized by Western blotting using anti KETYY antibody. Each reaction was performed in the presence of Tris buffer with the indicated pH values. Representative of four different experiments is shown. The right labels indicate the symbols of cleavage sites introduced by Jørgensen [29], together with the new T4 site. Note that the very faint bands appearing at 4 kDa in the presence of Na+ or K+ likely represent conformational fluctuations of the protein during incubation with the protease (Ref. [58]).