Abstract
Bidirectional cancer-promoting and anti-cancer effects of arsenic for cancer cells have been revealed in previous studies. However, each of these effects (cancer-promoting or anti-cancer) was found in different cells at different treated-concentration of arsenic. In this study, we for the first time indicated that arsenic at concentration of 3 µM, equal to average concentration in drinking water in cancer-prone areas in Bangladesh, simultaneously expressed its bidirectional effects on human squamous cell carcinoma HSC5 cells with distinct pathways. Treatment with 3 µM of arsenic promoted cell invasion via upregulation of expression of MT1-MMP and downregulation of expression of p14ARF and simultaneously induced cell apoptosis through inhibition of expression of N-cadherin and increase of expression of p21(WAF1/CIP1) at both transcript and protein levels in HSC5 cells. We also showed that inhibition of MT1-MMP expression by NSC405020 resulted in decrease of arsenic-mediated invasion of HSC5 cells involving decrease in phosphorylated extracellular signal-regulated kinases (pERK). Taken together, our biological and biochemical findings suggested that arsenic expressed bidirectional effects as a carcinogen and an anti-cancer agent in human squamous cell carcinoma HSC5 cells with distinct pathways. Our results might play an important scientific evident for further studies to find out a better way in treatment of arsenic-induced cancers, especially in squamous cell carcinoma.
Introduction
Arsenic contamination in drinking well water is a serious public health problem in the world [1], [2]. Arsenic is a well-documented human carcinogen. Chronic low-dose exposure of arsenic caused skin discoloration, chronic indigestion, hypertension, peripheral vascular disease, ischemic heart disease, and many types of cancers including skin, lung, bladder, liver, and kidney cancers [3]. Effects of arsenic as an agent for carcinogenesis or tumor progression or an anti-tumor drug depend on its concentration [4]–[6], duration of exposure [6], [7] and cancer cell types [3]–[7]. Previous auto radiographic animal studies showed that cutaneous squamous cell carcinoma (SCC) is one of the representative arsenic-mediated cancers [8]. Although arsenic has been widely recognized as a carcinogen, it also has been clinically used as an effective chemotherapeutic agent in treatment of leukemia in humans [9]. Arsenic also expressed its anti-cancer effects on various solid cancers, including cutaneous carcinoma, through promoting apoptotic cell death [10], [11]. The bidirectional cancer-promoting and anti-cancer effects of arsenic on cancer cells have led to a difficult situation to clarify the mechanism of arsenic-mediated cancer. Although there were many studies focusing on revealing the mechanism of effects of arsenic on cancer cells, it is still unclear.
Arrest of the cell cycle and apoptosis are related to cell death and to be considered as important ways for cancer treatment [12]–[15]. Previous studies showed that arsenic induces apoptosis in cancer cells via activation of expression of tumor suppressors of p21(WAF1/CIP1) and p14ARF (p19ARF in mouse) [12]–[15]. p21(WAF1/CIP1) and p14ARF play important roles in controlling the cell cycle arrest by regulating the activity of cyclins and cyclin-dependent kinases (CDK) [16]–[19]. p21(WAF1/CIP1) and p14ARF are able to inhibit cell growth through cell cycle arrest of skin cancer cells including melanoma, squamous cell carcinoma and basal cell carcinoma [20]–[24]. Other reports revealed that exposure to arsenic cause cell transformation through the inhibition of both protein and gene expression of the tumor suppressors p19ARF [22]–[24].
Invasion is hall mark for malignancy grade of cancer cells. It is reported that arsenic reduces the invasive and metastatic properties of glioma tumor cells via inhibition of activation of matrix metalloproteinase-14 (MT1-MMP) [7], [25], which is able to drive invasion of cancer cells largely by degrading ECM barriers. MT1-MMP is also considered as a upstream of ERK. ERK is a key molecule in the major signaling cassettes of the mitogen-activated protein kinase pathway [26], . ERK plays an important role in cancer development, [26], [27]. Thus MT1-MMP may be able to regulate the phosphorylated level of ERK [26], [27]. However, in the other studies, high concentration of arsenic enhances MT1-MMP expression in fibroblast cells [28]–[30]. Previous studies also reported that arsenic reduces expression of E-cadherins [31], [32]. Downregulation of E-cadherin is correlated with upregulation of N-cadherin, an invasion promoter molecule [33]–. N-cadherin–dependent adhesion impairs the upregulation of the cyclin-dependent kinase inhibitor p21 [37], [38]. Ectopic expression of N-cadherin increases tumor cell motility [35], [39].
Our previous report showed that there is about 3 µM (210.7 µg/L) of arsenic in the arsenic-polluted drinking well water (n = 72) in cancer-prone areas in Bangladesh [40]. There are many types of cancers including squamous cell carcinomas (SCC) occurring in these areas [40]. In this study, we for the first time showed that arsenic at concentration of 3 µM simultaneously acted as cancer-promoter and anti-cancer drug in human squamous cell carcinoma HSC5 cells with distinct pathways.
Materials and Methods
Reagents
Sodium arsenide (arsenic) was purchased from Sigma. NSC405020 was purchased from Millipore. Arsenic was dissolved in water for use. NSC405020 was dissolved in DMSO for use.
Cell culture
Human transformed keratinocytes HSC5 cells [40] (Health Science Research Resources Bank, Japan) were cultured in RPMI-1640, supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin/Streptomycin at 37°C in 5% CO2.
Crystal violet assay
Crystal violet assay was performed using the method previously described [40]. Briefly, cells (3×104 cells) were plated in six-well plates and cultured for 24 h. Cells were then treated with arsenic and cultured for a further 3 days. The viable adherent cells were fixed with 10% formalin and stained with 0.1% crystal violet. Absorbance at 595 nm in the stained cells solubilized with 0.1% SDS was measured using a microplate reader.
Invasion assay
Cell invasion ability was evaluated by invasion assay according to the method previously reported [41]. Briefly, 2×105 cells in 300 ml culture medium with 0.5% FBS were applied to the matrigel-coated upper chamber of 8 mm in diameter (8 mm in pore size). Then the upper chambers were placed in 24-well culture plates containing 600 ml conditioned medium with 0.5% FBS to trigger invasion activity and were incubated for 12 hours. Invading cells were stained with hematoxyline-eosine or crystal violet and counted under a microscope.
Real-time PCR analysis
Total RNA was prepared from cell line samples using a High Pure RNA Kit (Roche Diagnostics) according to the method previously described [41]. cDNA was then synthesized by reverse transcription of total RNA using Super-criptTMIII reverse transcriptase included in the RT enzyme mix and RT reaction mix according to the protocol previously described [41]. Real-time quantitative RT-PCR with SYBR green was performed using power SYBR1 Green PCR master mix (Applied Biosystems) in an ABI Prism7500 sequence detection system (Applied Biosystems). The expression levels of p14ARF, p21(WAF1/CIP1), MT1-MMP and N-cadherin transcripts measured by quantitative RT-PCR (real-time PCR) were adjusted through the transcript expression level of TATA-box-binding protein (TBP). PCR was carried out using 10 ml of power SYBR1 Green PCR master mix (Applied Biosystems) containing 900 nM forward primer and 900 nM reverse primer in a final volume of 20 ml. Sequences of primers are presented as below:
Forward: GTGGTCTCGGACCATGTC
and Reverse: GTAGCCATATTGCTGTAGCC for MT1-MMP.
Forward: ATTGCTGTTTTGGACCGAGA
and Reverse: CACTTGAGGGGCATTGTCAT for N-cadherin.
Forward: AAGTCAGTTCCTTGTGGAGC
and Reverse: ATTAGCGCATCACAGTCGCG for p21(WAF1/CIP1).
Forward: ATGGTGCGCAGGTTCTTGGT
and Reverse: TGCCCATCATCATGACCTGG for p14ARF.
Forward: CACGAACCACGGCACTGATT
and Reverse: TTTTCTTGCTGCCAGTCTGGAC for TBP.
Immunoblot analysis
Immunoblot analysis was performed according to the method described previously [42]. Rabbit polyclonal first antibodies against phosphorylated threonine 202 in ERK1 and phosphorylated tyrosine 204 in ERK2 (Cell Signaling), anti-matrix metalloproteinase-14 (MT1-MMP) hinge region antibody (Millipore), ERK1/2 (Cell Signaling), Goat polyclonal antibodies against p14ARF and p21(WAF1/CIP1) (Santa Cruz), mouse monoclonal antibody against N-cadherin (BD Biosciences ) and mouse monoclonal antibody against alpha-TUBULIN (SIGMA).
Statistical analysis
Statistical analysis in this study was performed according to the method previously described [40]. Results from three independent experiments in each group were statistically analyzed by Student's t-test. The SPSS (version 18) software package (SPSS Japan Inc.) was used for these statistical analyses, and the significance level was set at p<0.05.
Results
Arsenic induced apoptosis of HSC5 cells
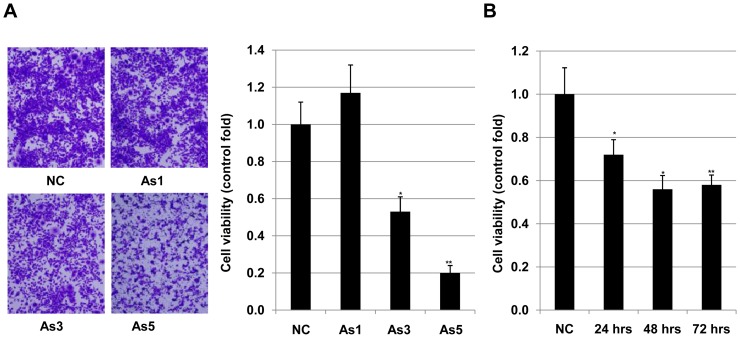
Our previous fieldwork showed that concentration of arsenic in arsenic-polluted drinking water in cancer-prone areas in Bangladesh is about 3 µM (210.7 µg/L) [40]. However, there is a fact that it is difficult to identify the effect of arsenic on cancer development [3]–[11] therefore we decided to investigate of effects of arsenic on apoptosis of HSC5 cells. HSC5 cells were treated with arsenic at 0, 1.0, 3.0, 5.0 and 10.0 µM for 24 hrs. It showed that the higher concentration of arsenic caused the stronger apoptosis of the cells. At concentration of 1 µM, arsenic had no effect on cell dead (Figure 1A). However, at 3 µM and 5 µM, arsenic caused decrease of cell viability almost 2 and 5 folds, respectively (Figure 1A). 10 µM of arsenic led to almost all cells dead (data not shown). We then investigated the effect of 3 µM of arsenic on the dead of HSC5 cells with a time course. Treatment with arsenic for 24 hours, 48 hours and 72 hours significantly decreased viability of HSC5 cells 1.4, 1.8 and 1.7 times, respectively (Figure 1B). These results indicated that apoptosis of HSC5 cells were directly caused by arsenic.
Figure 1. Effects of arsenic on apoptosis of HSC5 cells.
Viability of HSC5 cells treated with 0, 1.0, 3.0 and 5.0 µM of arsenic was evaluated by crystal violet (CV). Cells were presented in photographs (A) and ratios of arsenic treated live cells and control live cells were presented in a graph (B). **, Significantly different (p<0.01) from the control by the Student's t-test.
Arsenic promoted invasion of HSC5 cells
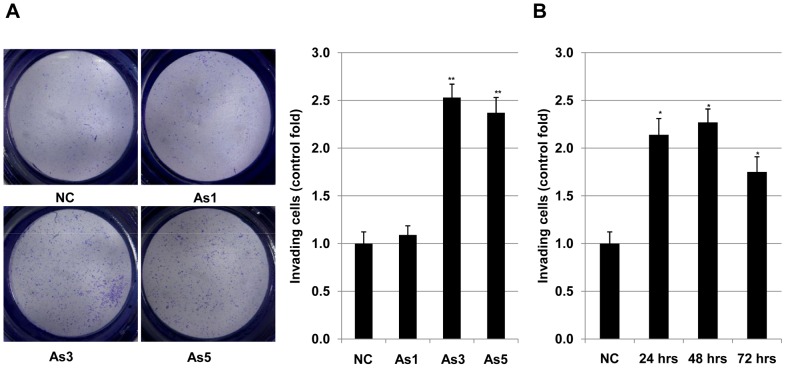
We next examined effects of arsenic on invasion of HSC5 cells. Cells were pre-treated with arsenic at different concentrations (0, 1.0, 3.0, 5.0 and 10.0 µM) for 24 hours before harvesting for invasion assay. Treatment with 1 µM of arsenic had no effect, however 3 µM and 5 µM promoted invasion of HSC5 about 1.8 and 3.7 folds, respectively (Figure 2A). At 10 µM, arsenic caused the dead of almost all cells, therefore it was not possible to investigate the effect of arsenic on invasion of cell at this concentration. We then investigated the effect of 3 µM of arsenic on invasion of HSC5 cells with a time course of 0, 24, 48 and 72 hours. Pre-treated with arsenic for 24, 48 and 72 hours increased invasion of HSC5 cells 2.14, 2.27 and 1.75 folds, respectively (Figure 2B). There results suggested that arsenic resulted in promotion of invasion of HSC5 cells. Treatment with 3 µM or 5 µM of arsenic increased apoptosis (Figure 1) and simultaneously promoted invasion of HSC5 cells (Figure 2). These results are scientific evidents for bifunctions of arsenic as a carcinogen and anti-cancer agent in cancer squamous cell carcinoma HSC5 cells. Basing on these results and the data from the fieldwork, we decided to use 3 µM of arsenic for further experiments.
Figure 2. Effects of arsenic on invasion of HSC5 cells.
Invasive ability of of HSC5 cells treated 0, 1.0, 3.0 and 5.0 µM of arsenic were evaluated by invasion assay. Number of invading HSC5 cells treated with arsenic in the invasion assay were presented in photographs (A) and a graph (B). **, Significantly different (p<0.01) from the control by the Student's t-test.
Arsenic promoted invasion of HSC5 cells by upregulation of MT1-MMP and downregulation of p14RAF at both transcript and expression levels
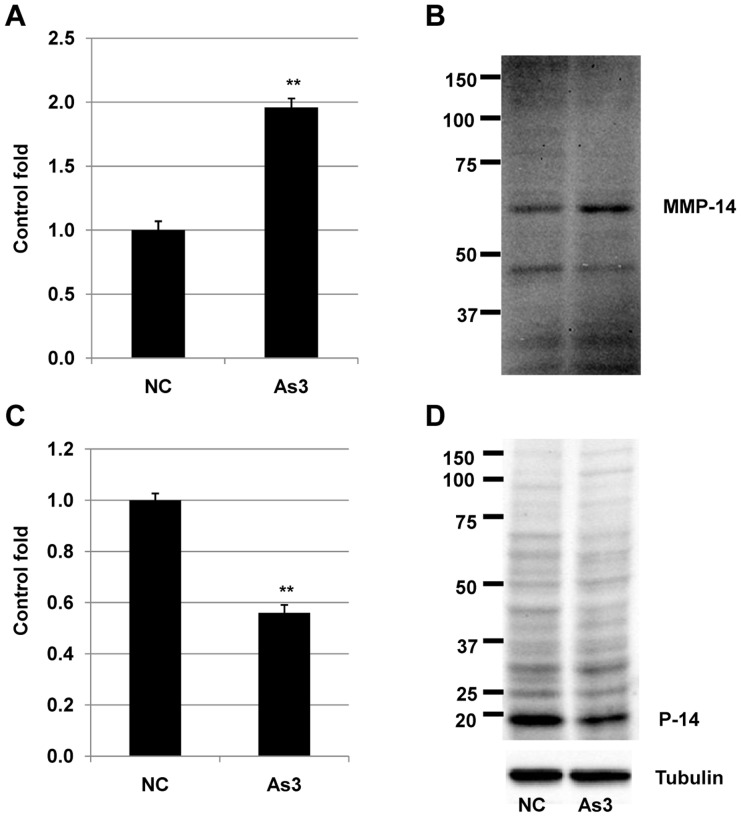
We next examined the molecular mechanism of arsenic-mediated cellular invasion in HSC5 cells. Treatment with 3 µM of arsenic induced transcript and expression levels of MT1-MMP (Figure 3A-B) and inhibited transcript and expression levels of p14ARF (Figure 3C-D). Previous studies [20]–[24], [27] indicated that MT1-MMP and p14ARF might play important roles in modulating growth and invasion of squamous cell carcinoma cells. In accordance with these previous reports [20]–[24], [27], our results suggested that arsenic might promote invasion of HSC5 cells via upregulation of MT1-MMP and downregulation of p14ARF.
Figure 3. MT1-MMP and P14 transcript and protein expression levels in HSC5 cells treated with arsenic.
A and C) transcript expression levels of MT1-MMP and p14 were measured by real-time PCR. **, significantly different (p<0.01) from the control by Student's t-test. B and D) Protein expression levels of MT1-MMP and p14 were measured by immunoblot. TUBULIN was used as a positive control. Three independent experiments were performed and the same results were obtained.
Arsenic induced apoptosis of HSC5 cells by downregulation of N-cadherin and and upregulation of p21(WAF1/CIP1) at both transcript and expression levels
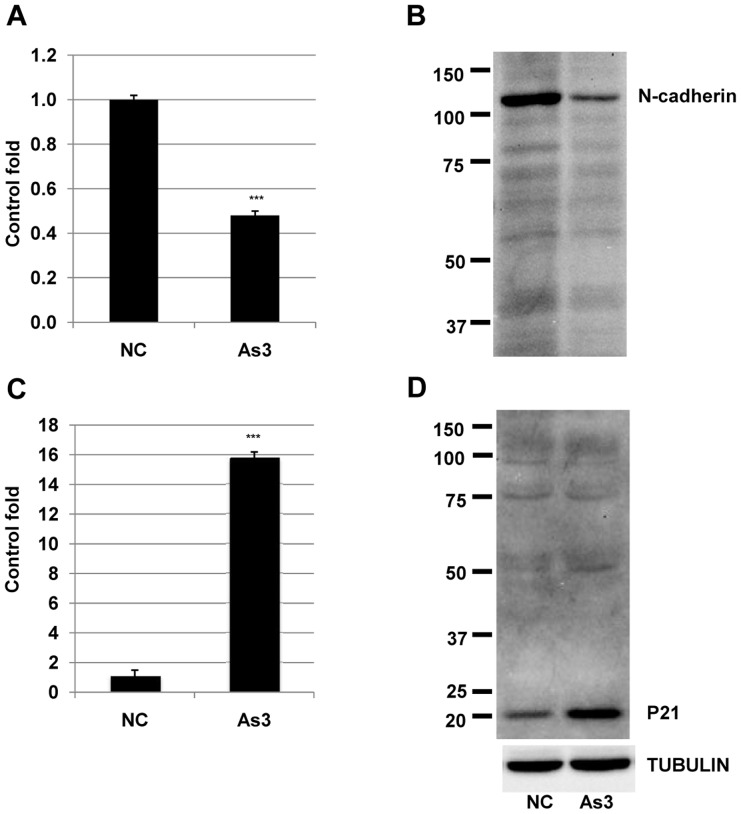
We then investigated the molecular mechanism of arsenic-mediated apoptosis in HSC5 cells. Treatment with 3 µM of arsenic strongly reduced transcript and expression levels of N-cadherin (Figure 4A-B) and promoted transcript and expression levels of p21(WAF1/CIP1) (Figure 4C-D). Previous studies [20]–[24], [29]–[36] showed that N-cadherin and p21(WAF1/CIP1) might play important roles in modulating of apoptosis of human squamous cell carcinoma cells. Our results in this study suggested that arsenic might cause apoptosis of HSC5 cells through downregulation of N-cadherin and/or upregulation of p21(WAF1/CIP1).
Figure 4. N-cadherin and p21 transcript and protein expression levels in HSC5 cells treated with arsenic.
A and C) transcript expression levels of N-cadherin and p21 were measured by real-time PCR. ***, significantly different (p<0.001) from the control by Student's t-test. B and D) Protein expression levels of N-cadherin and p21 were measured by immunoblot. TUBULIN was used as a positive control. Three independent experiments were performed and the same results were obtained.
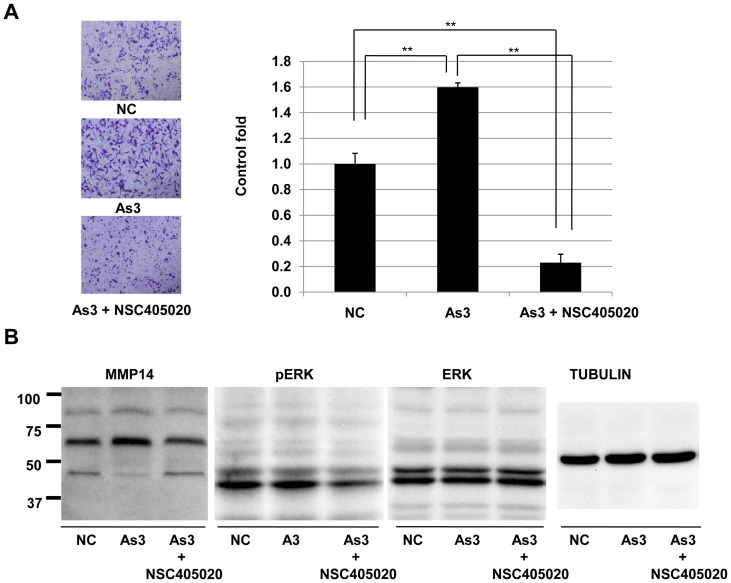
Inhibition of arsenic-mediated promotion of invasion HSC5 cells by a MT1-MMP inhibitor
We next examined the effect of NSC405020, a MT1-MMP inhibitor, on arsenic-mediated invasion of HSC5 cells (Figure 5). Since MT1-MMP has been reported to be potential sited upstream of ERK [41], [42] and may be associated with arsenic-mediated invasion (Figure 2). Treatment with 3 µM arsenic again increased invasion (Figure 5A) with an increase in expression level of MT1-MMP (Figure 5B). However, there was no change in the phosphorylated level of ERK (pERK). These results indicated that the bidirectional effects of arsenic at this concentration on pERK were balance. Arsenic-mediated invasion was blocked by treatment with 1 µM of NSC405020 (Figure 5A). NSC405020 (1 µM) caused to decrease of expression level of MT1-MMP as well as decrease of phosphorylated of ERK in HSC5 cells (Figure 5B).
Figure 5. Effects of arsenic on invasive activity and phosphorylation and/or expression levels of MT1-MMP and ERK 4 in HSC5 cells.
A), Invasive activity of HSC5 treated with 3 µM of arsenic was evaluated invasion assay. Level of invasive ability is presented as number of invading cells in a graph (left) and photographs (right). **, Significantly different (p<0.01) from the control by the Student's t-test. Phosphorylated levels of ERK (P-ERK) and protein expression levels of MT1-MMP and ERK in HSC5 cells treated with 3 µM arsenic for 24 hours are presented. TUBULIN protein expression levels are presented as an internal control.
Discussion
Arsenic has been considered as an agent for carcinogenesis and tumor progression for a long time ago [1], [2]. Based on the analysis of 52,202 hand tube-well water samples during the last 14 years in Bangladesh showed that around 36 million and 22 million people could be drinking As-contaminated water above 10 and 50 µg/l, respectively [43]. This may be the main cause for serious problem of cancer development, especially for skin cancer in Bangladesh [3]. In a contrast way, arsenic also has been used as a drug for treatment of many types of cancers [8]–[11]. Basing on our previous result [40], we examined the effects of 3 µM arsenic on cellular invasion, a hallmark of malignancy grade of cancer cell and apoptosis, a marker for cell dead in cancer treatment. Our result showed that arsenic simultaneously strongly induced apoptosis (Figure 1) and promoted invasion (Figure 2) of HSC5 cells. These results suggested that at this concentration, arsenic expressed its bidirectional functions in squamous cell carcinoma HSC5 cells. We then investigated the molecular mechanisms related to these events in HSC5 cells. Our results revealed that 3 µM arsenic enhanced cellular invasion through upregulation of membrane type 1 matrix metalloproteinase (MT1-MMP) (Figure 3A), which plays crucial roles in tumorigenesis [7], and downregulation of p14ARF (Figure 3B), which is an inhibitor for cell proliferation [20]–[24] at both transcript and expression levels. This is the first time we showed that there is a possibility in cooperation between MT1-MMP and p14ARF in cancer development in HSC5 cells. In the other hand, our results showed that 3 µM arsenic induced apoptosis of HSC5 cells via downregulation of N-cadherin (Figure 4A), which plays role in cell differentiation, transformation, as well as invasion [33]–[36] and upregulation p21(WAF1/CIP1) (Figure 4B), a tumor suppressor [20]–[24] at both transcript and expression levels. Our results in accordance with previous studies [37], [38] suggested that N-cadherin might participate to the associated-cell cycle arrest through the nuclear accumulation of cyclin-dependent kinase inhibitors p21. Although both p14ARF and p21(WAF1/CIP1) were reported as molecules which play important roles in controlling the cell cycle arrest by regulating the activities of cyclins and cyclin-dependent kinases (CDK) [16]–[19], in this study, these molecules expressed their distinct effects on invasion and apoptosis of HSC5 cells. Further, we showed that NSC405020, a MT1-MMP inhibitor, inhibited the arsenic-mediated promotion of invasion HSC5 cells (Figure 5). It can be explained that treatment with NSC405020 resulted in decreasing of expression level of MT1-MMP. In turn, MT1-MMP regulated the phosphorylated level of extracellular signal-regulated kinases (pERK) [26], [27], which plays an important role in cellular invasion, proliferation and tumor development [26], [27]. Finally, downregulation of MT1-MMP and pERK by NSC405020 led to decreasing the invasion of HSC5 cells (Figure 5). Bidirectional cancer-promoting and anti-cancer effects of arsenic for cancer cells cells have been revealed. However, arsenic as a cancer-promoting agent or an anti-cancer drug was found in different cells at different treated-concentration in a particular report [1]–[3], [8]–[11]. This is the first time we simultaneously showed the bidirectional functions of arsenic in human squamous cell carcinoma HSC5 cells with distinct molecular pathways. This study helped to explain the reason why although there are many people have been exposed to arsenic, not all of them have arsenicosis diseases including cancers. Our results provided an important information for other studies in the future to find out a better way in treatment of arsenic-induced cancers, especially in squamous cell carcinoma.
Funding Statement
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-NN.02-2013.07. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, et al. (2000) Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect 108: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg M, Stengel C, Pham TK, Pham HV, Sampson ML, et al. (2007) Magnitude of arsenic pollution in the Mekong and Red River Deltas-Cambodia and Vietnam. Sci Total Environ 372: 413–425. [DOI] [PubMed] [Google Scholar]
- 3. Gebel TW (1999) Arsenic and drinking water contamination. Science 283: 1458–1459. [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Ling M, Xu Y, Wang S, Li Z, et al. (2010) The repressive effect of NF-kappaB on p53 by mot-2 is involved in human keratinocyte transformation induced by low levels of arsenite. Toxicol Sci 116: 174–182. [DOI] [PubMed] [Google Scholar]
- 5. Pi J, Diwan BA, Sun Y, Liu J, Qu W, et al. (2008) Arsenic-induced malignant transformation of human keratinocytes: involvement of Nrf2. Free Radic Biol Med 45: 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu ZM, Huang HS (2008) Arsenic trioxide phosphorylates c-Fos to transactivate p21(WAF1/CIP1) expression. Toxicol Appl Pharmacol 233: 297–307. [DOI] [PubMed] [Google Scholar]
- 7. Kato M, Hossain K, Iida M, Sato H, Uemura N, et al. (2008) Arsenic enhances matrix metalloproteinase-14 expression in fibroblasts. J Toxicol Environ Health A 71: 1053–1055. [DOI] [PubMed] [Google Scholar]
- 8. Lansdown AB (1995) Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Crit Rev Toxicol 25: 397–462. [DOI] [PubMed] [Google Scholar]
- 9. Lunghi P, Costanzo A, Levrero M, Bonati A (2004) Treatment with arsenic trioxide (ATO) and MEK1 inhibitor activates the p73–p53 AIP1 apoptotic pathway in leukemia cells. Blood 104: 519–525. [DOI] [PubMed] [Google Scholar]
- 10. Huang HS, Chang WC, Chen CJ (2002) Involvement of reactive oxygen species in arsenite-induced downregulation of phospholipid hydroperoxide glutathione peroxidase in human epidermoid carcinoma A431 cells. Free Radic Biol Med 33: 864–873. [DOI] [PubMed] [Google Scholar]
- 11. Jiang XH, Wong BC, Yuen ST, Jiang SH, Cho CH, et al. (2001) Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int J Cancer 91: 173–179. [DOI] [PubMed] [Google Scholar]
- 12. Huang HS, Liu ZM, Cheng YL (2011) Involvement of glycogen synthase kinase-3β in arsenic trioxide-induced p21 expression. Toxicol Sci 121: 101–109. [DOI] [PubMed] [Google Scholar]
- 13. Kim YJ, Chung JY, Lee SG, Kim JY, Park JE, et al. (2011) Arsenic trioxide-induced apoptosis in TM4 Sertoli cells: the potential involvement of p21 expression and p53 phosphorylation. Toxicology 285: 142–151. [DOI] [PubMed] [Google Scholar]
- 14. Hassani S, Ghaffari SH, Zaker F, Mirzaee R, Mardani H, et al. (2013) Azidothymidine hinders arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells by induction of p21 and attenuation of G2/M arrest. Ann Hematol 92: 1207–1020. [DOI] [PubMed] [Google Scholar]
- 15. Kim HG, Kim DG, Li S, Lee KY, Li X, et al. (2012) Polycomb (PcG) Proteins, BMI1 and SUZ12, regulate arsenic-induced cell transformation. J Biol Chem 287: 31920–31928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bottazzi ME, Zhu X, Bohmer RM, Assoian RK (1999) Regulation of p21(cip1) expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J Cell Biol 146: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roovers K, Assoian RK (2000) Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22: 818–826. [DOI] [PubMed] [Google Scholar]
- 18. Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9: 1149–1163. [DOI] [PubMed] [Google Scholar]
- 19. Torii S, Yamamoto T, Tsuchiya Y, Nishida E (2006) ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci 97: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang M, Li J, Wang L, Tian Z, Zhang P, et al. (2013) Prognostic significance of p21, p27 and survivin protein expression in patients with oral squamous cell carcinoma. Oncol Lett 6: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiang KC, Yeh CN, Hsu JT, Chen LW, Kuo SF, et al. (2013) MART-10, a novel vitamin D analog, inhibits head and neck squamous carcinoma cells growth through cell cycle arrest at G0/G1 with upregulation of p21 and p27 and downregulation of telomerase. J Steroid Biochem Mol Biol 138: 427–434. [DOI] [PubMed] [Google Scholar]
- 22. Ling Y, Zhang C, Shen R, Xu Y, Zhu C, et al. (2014) p14ARF repression induced by promoter methylation associated with metastasis in esophageal squamous cell carcinoma. Dis Esophagus 27: 182–187. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Sturgis EM, Zafereo ME, Wei Q, Li G (2011) p14ARF genetic polymorphisms and susceptibility to second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Cancer 117: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng TH, Hsu PK, Li AF, Hung IC, Huang MH, et al. (2009) Correlation of p53, MDM2 and p14(ARF) protein expression in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 135: 1577–1582. [DOI] [PubMed] [Google Scholar]
- 25. Lin TH, Kuo HC, Chou FP, Lu FJ (2008) Berberine enhances inhibition of glioma tumor cell migration and invasiveness mediated by arsenic trioxide. BMC Cancer 8: 58 doi:10.1186/1471-2407-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, et al. (2003) Differential regulation of cell motility and invasion by FAK. J Cell Biol 160: 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takino T, Tsuge H, Ozawa T, Sato H (2010) MT1-MMP promotes cell growth and ERK activation through c-Src and paxillin in three-dimensional collagen matrix. Biochem Biophys Res Commun 396: 1042–1047. [DOI] [PubMed] [Google Scholar]
- 28. Ota I, Li XY, Hu Y, Weiss SJ (2009) Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci USA 106: 20318–20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabeh F, Shimizu-Hirota R, Weiss SJ (2009) Protease-dependent versus independent cancer cell invasion programs: three-dimensional amoeboid movement revisite. J Cell Biol 185: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sugiyama N, Varjosalo M, Meller P, Lohi J, Hyytiäinen M, et al. (2010) Fibroblast growth factor receptor 4 regulates tumor invasion by coupling fibroblast growth factor signaling to extracellular matrix degradation. Cancer Res 70: 7851–7861. [DOI] [PubMed] [Google Scholar]
- 31. Nriagu J, Lin TS, Mazumder DG, Chatterjee D (2012) E-cadherin polymorphisms and susceptibility to arsenic-related skin lesions in West Bengal, India. Sci Total Environ 420: 65–72. [DOI] [PubMed] [Google Scholar]
- 32. Yu J, Qian H, Li Y, Wang Y, Zhang X, et al. (2007) Arsenic trioxide (As2O3) reduces the invasive and metastatic properties of cervical cancer cells in vitro and in vivo. Gynecol Oncol 106: 400–406. [DOI] [PubMed] [Google Scholar]
- 33. Aplin AE, Howe AK, Juliano RL (1999) Cell adhesion molecules, signal transduction and cell growth. Curr Option Cell Biol 11: 737–744. [DOI] [PubMed] [Google Scholar]
- 34. Johnson J (1999) Cell adhesion molecules in the development and progression of malignant melanoma. Cancer and Metastasis Rev 18: 345–357. [DOI] [PubMed] [Google Scholar]
- 35. Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA (2000) Exogenous expression of N-cadherin in breast cancer cell induces cell migration, invasion and metastasis. J Cell Biol 148: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Derycke LDM, Bracke ME (2004) N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signaling. Int J Dev Biol 48: 463–476. [DOI] [PubMed] [Google Scholar]
- 37. Liu P, Yang J, Pei J, Pei D, Wilson MJ (2010) Regulation of MT1-MMP activity by β-catenin in MDCK non-cancer and HT1080 cancer cells. J Cell Physiol 225: 810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ciołczyk-Wierzbicka D, Gil D, Laidler P (2012) The Inhibition of Cell Proliferation Using Silencing of N-Cadherin Gene by siRNA Process in Human Melanoma Cell Lines. Curr Med Chem 19: 145–151. [DOI] [PubMed] [Google Scholar]
- 39. Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ (1999) N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 147: 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yajima I, Uemura N, Nizam S, Md Khhalequzzaman, Thang ND, et al. (2012) Barium inhibits arsenic-mediated apoptotic cell death in human squamous cell carcinoma cells. Arch Toxicol 86: 961–973. [DOI] [PubMed] [Google Scholar]
- 41. Thang ND, Yajima I, Kumasaka MY, Ohnuma S, Yanagishita T, et al. (2011) Barium Promotes Anchorage-Independent Growth and Invasion of Human HaCaT Keratinocytes via Activation of c-SRC Kinase. PLoS ONE 6(10): e25636 doi:10.1371/journal.pone.0025636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thang ND, Yajima I, Nakagawa K, Tsuzuki T, Kumasaka MY, et al. (2012) A novel hairless mouse model for malignant melanoma. J Dermatol Sci 65: 207–212. [DOI] [PubMed] [Google Scholar]
- 43. Chakraborti D, Rahman MM, Das B, Murrill M, Dey S, et al. (2010) Status of groundwater arsenic contamination in Bangladesh: a 14-year study report. Water Res 44: 5789–802. [DOI] [PubMed] [Google Scholar]