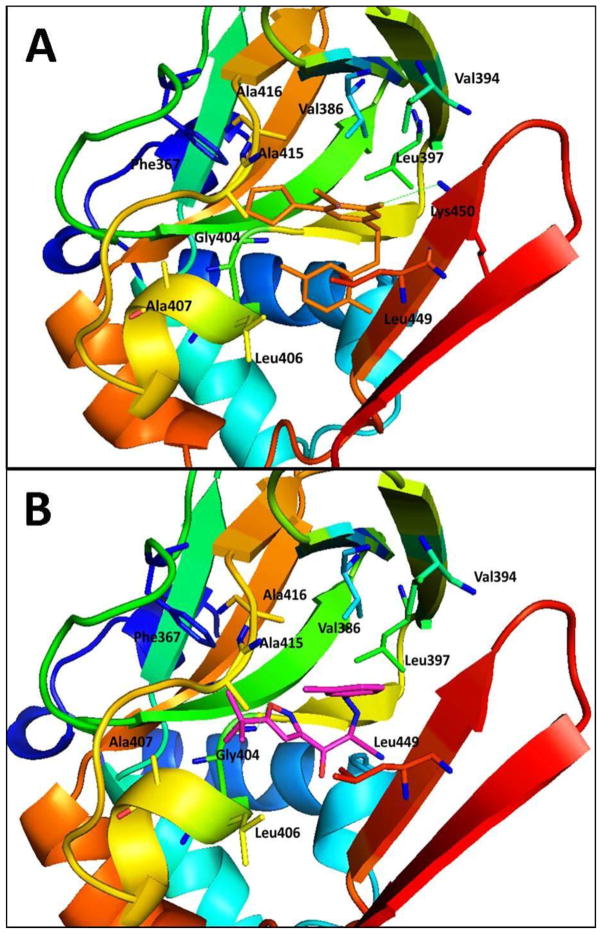
Figure 11.
Predicted binding mode and molecular docking of 25, and 23 into the cAMP binding domain B (CNBD-B) of EPAC2 protein. Important residues are drawn in sticks. Hydrogen bonds are shown as dashed green lines. (A) Binding mode of 25 (orange). The cyclopentyl group at the C-6 position interacts with the hydrophobic residues of Phe367, Ala415 and Ala416, while the hydrophobic S-benzyl moiety of 2-position forms interactions with Leu406 and Leu449. (B) Binding mode of 23 (pink). The tert-butylisoxazolyl moiety forms a hydrogen bond with the residue Gly404 and interacts with the hydrophobic residues of Phe367, Leu406, Ala407, and Ala415. Meanwhile, 3-chlorophenyl fragment forms hydrophobic interactions with residues Val386, Val394 and Leu397.