SUMMARY
Thermogenesis, the production of heat energy, is the specific, neurally-regulated, metabolic function of brown adipose tissue (BAT) and contributes to the maintenance of body temperature during cold exposure and to the elevated core temperature during several behavioral states, including wakefulness, the acute phase response (fever), and stress. BAT energy expenditure requires metabolic fuel availability and contributes to energy balance. This review summarizes the functional organization and neurochemical influences within the CNS networks governing the level of BAT sympathetic nerve activity to produce the thermoregulatory and metabolically-driven alterations in BAT thermogenesis and energy expenditure that contribute to overall energy homeostasis.
Keywords: Brown adipose tissue, thermogenesis, fever, thermoregulation, energy expenditure, sympathetic nerve activity, preoptic hypothalamus, rostral raphe pallidus, dorsomedial hypothalamus, paraventricular hypothalamus
INTRODUCTION
Thermogenesis, the production of heat energy, occurs to a greater or lesser extent in all tissues, since heat generation is an unavoidable consequence of the inefficiency of both mitochondrial adenosine triphosphate (ATP) production and cellular ATP utilization. However, thermogenesis is the specific metabolic function of beige and brown adipose tissue (BAT) in many species from mouse to man and is accomplished by the heat generating capacity of a ‘proton leak’ across the extensive mitochondrial membranes of the beige and brown adipocytes, facilitated by the high expression of uncoupling protein-1 (UCP1) in BAT mitochondria (Cannon and Nedergaard, 2004). BAT thermogenesis is an essential component of the homeostatic repertoire to maintain body temperature during the challenge of low environmental temperature. The heat generated during pyrogen (i.e., fever-producing substances)-stimulated thermogenesis in BAT also contributes to fever, a controlled elevation in body temperature that reduces pathogen viability and stimulates immune cell responses. However, since energy consumption during BAT thermogenesis involves oxidation of lipid and glucose fuel molecules, not only is BAT thermogenesis potently influenced in a permissive manner by signals related to fuel substrate and oxygen availability, but also, the level of BAT thermogenesis can contribute to energy balance, regulation of body adipose stores and glucose utilization. Indeed, with the recent confirmation of metabolically-active BAT in adult humans (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009), there is increasing interest in devising pharmacological approaches to activate BAT as a metabolic furnace to burn the excess calories stored in the white adipose tissue of the obese. This will require not only therapeutic strategies to augment BAT depots, but also those to increase the CNS sympathetic drive to BAT, the latter requiring an improved understanding of the CNS mechanisms integrating the wide array of signals that influence BAT energy expenditure and overall energy homeostasis. This review will summarize our understanding of the functional organization and neurochemical influences specifically within the CNS networks that modulate BAT thermogenesis and BAT energy expenditure by altering the level of BAT sympathetic nerve activity (SNA) and thus the norepinephrine release onto β3-adrenergic receptors in brown adipocyte membranes.
The level of BAT sympathetic outflow is determined primarily by 3 factors. BAT is principally a thermoeffector and the core thermoregulatory network in the CNS (Fig. 1 and reviewed in (Morrison et al., 2012)) comprises the fundamental pathways through which cutaneous and visceral cold and warm sensation and/or reductions or elevations in brain temperature elicit changes in BAT thermogenesis to protect against or to counter changes in the temperature of the brain and other critical tissues. This circuit, involving thermal afferent pathways, hypothalamic sensorimotor integration and descending efferent pathways to the spinal BAT sympathetic preganglionic neurons, provides an important framework for understanding the overall regulation of BAT thermogenesis by the CNS. Secondly, a variety of behavioral states, including wakefulness, immunologic responses, and stress, are characterized by elevations in body temperature to which central command-driven BAT activation makes a significant contribution. Although the neural circuitry and transmitters underlying behavioral state modulations of BAT are poorly understood, it is likely that at least some of the neurochemical influences (e.g., histamine and orexin) and modulatory brain regions depicted in Figure 1 are related to such behavioral state controls on BAT thermogenesis. Thirdly, since the high metabolic rate of BAT during thermogenesis cannot be sustained without a dependable supply of metabolic fuels, particularly oxygen, lipolytic by-products and glucose, the CNS network driving cold-defensive and behavioral BAT activation is strongly influenced by signals reflecting the short- and long-term availability of the fuel molecules essential for BAT metabolism. Synaptic and hormonal signals related to metabolic substrates can influence the sympathetic outflow to BAT in several ways. Signals that increase as the availability of a metabolic substrate falls can produce a potent inhibition of BAT sympathoexcitatory neurons, as is the case with arterial chemoreceptor inputs during systemic hypoxia (Madden and Morrison, 2005). In contrast, a tonically-active signal such as leptin, indicating the availability of a lipid fuel store in positive balance, may act within the CNS network for BAT activation in a ‘permissive’ manner by reducing a tonic inhibition of BAT activity (Kong et al., 2012) or by facilitating the discharge of BAT sympathoexcitatory neurons (Zhang et al., 2011a). Although several of these modulatory influences on the CNS network for BAT activation are recognized, in most cases, little is known about the pathways and neurochemical mediators through which they influence BAT activity. Thus, they are likely included in the modulatory (i.e., non-thermoregulatory) influences on BAT activity summarized in Figure 1, which indicate not only the complexity of the central control of this highly metabolic organ, but also the many central mechanisms determining BAT sympathetic outflow that remain to be explored.
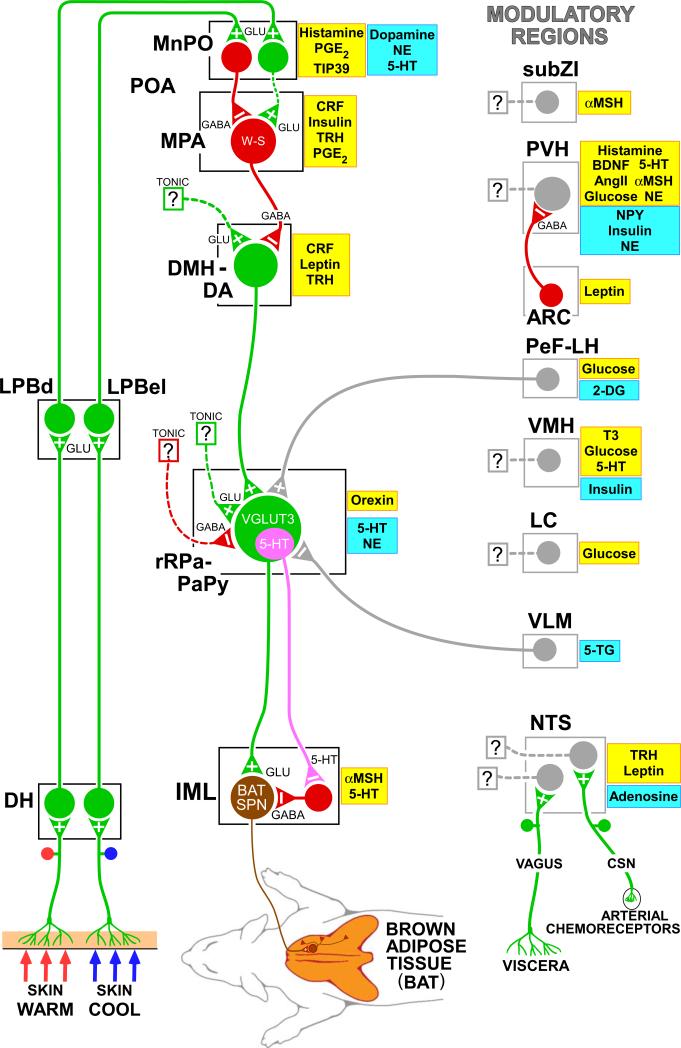
Figure 1. Model for the neuroanatomical and neurotransmitter/hormonal organization of the core thermoregulatory network and other CNS sites controlling and modulating brown adipose tissue (BAT) thermogenesis.
Cool and warm cutaneous thermal sensory receptors transmit signals to respective primary sensory neurons in the dorsal root ganglia which relay this thermal information to second-order thermal sensory neurons in the dorsal horn (DH). Cool sensory DH neurons glutamatergically activate third-order sensory neurons in the external lateral subnucleus of the lateral parabrachial nucleus (LPBel), while warm sensory DH neurons project to third-order sensory neurons in the dorsal subnucleus of the lateral parabrachial nucleus (LPBd). Thermosensory signals for thermoregulatory responses are transmitted from the LPB to the preoptic area (POA) where GABAergic interneurons in the median preoptic (MnPO) subnucleus are activated by glutamatergic inputs from cool-activated neurons in LPBel and inhibit a BAT-regulating population of warm-sensitive (W-S) neurons in the medial preoptic area (MPA). In contrast, glutamatergic interneurons in the MnPO, postulated to be excited by glutamatergic inputs from warm-activated neurons in LPBd, excite W-S neurons in MPA. Prostaglandin (PG) E2 binds to EP3 receptors to inhibit the activity of W-S neurons in the POA. Preoptic W-S neurons providing thermoregulatory control of BAT thermogenesis inhibit BAT sympathoexcitatory neurons in the dorsomedial hypothalamus and dorsal hypothalamic area (DMH/DA) which, when disinhibited during skin cooling, excite BAT sympathetic premotor neurons in the rostral ventromedial medulla, including the rostral raphe pallidus (rRPa) and parapyramidal area (PaPy), that project to BAT sympathetic preganglionic neurons (SPN) in the spinal intermediolateral nucleus (IML). Some BAT premotor neurons can release glutamate (GLU) to excite BAT sympathetic preganglionic neurons and increase BAT sympathetic nerve activity, while others can release serotonin (5-HT) to interact with 5-HT1A receptors, potentially on inhibitory interneurons in the IML, to increase the BAT sympathetic outflow. Orexinergic neurons in the perifornical lateral hypothalamus (PeF-LH) project to the rRPa to increase the excitability of BAT sympathetic premotor neurons. Activation of neurons in the ventrolateral medulla (VLM) produces an inhibition of BAT thermogenesis, at least in part by noradrenergic activation of α2 receptors on rRPa neurons. Neurochemicals/hormones in yellow boxes activate and those in blue boxes reduce BAT activity. 2-DG, 2-deoxyglucose; 5-HT, 5-hydroxytryptamine; 5-TG, 5-thioglucose; αMSH, alpha melanocyte-stimulating hormone; AngII, angiotensin II; BDNF, brain-derived neurotrophic factor; CRF, corticotrophin releasing factor; CSN, carotid sinus nerve; NE, norepinephrine; NPY, neuropeptide Y; PGE2, prostaglandin E2; T3, triiodothyronine; TIP39, tuberoinfundibular peptide of 39 residues; TRH, thyrotropin-releasing hormone; VGLUT3, vesicular glutamate transporter 3. Copyright 2014 by Oregon Health and Science University.
SENSORY PATHWAYS AFFECTING BAT THERMOGENESIS
The membranes of thermal afferent neurons contain transient receptor potential (TRP) cation channels whose temperature-dependent conductances transduce skin temperature into primary thermoreceptor afferent neuronal activity. The TRPM8 channel, activated by menthol and cooling, is the primary candidate for the cutaneous cold receptor TRP channel. Some non-thermal, unmyelinated afferents expressing TRP channels also have access to central thermoregulatory circuits: endogenous ligand-stimulated TRPV1 channels inhibit BAT thermogenesis (Steiner et al., 2007), although intragastric capsiate, a TRP agonist, activates BAT (Ono et al., 2011). Treatment with capsaicin, a TRPV1 agonist that is the active component in chili peppers responsible for their evoked sensation of heat or pain, reduced systemic norepinephrine (NE)-stimulated BAT thermogenesis, as well as BAT temperature (TBAT) and core temperature (TCORE) following cold exposure (Vaughan and Bartness, 2012), consistent with a role for sensory inputs from BAT depots in modulating BAT thermogenesis.
Primary thermoreceptor dorsal root ganglion neurons deliver thermal information to lamina I neurons in the spinal (or trigeminal) dorsal horn (Craig, 2002), where the TRP channels in their central endings may also provide a substrate for spinal cord or trigeminal nucleus temperature to influence BAT thermogenesis. Spinal lamina I cold thermal responsive neurons provide a glutamatergic excitation to neurons in the external lateral subdivision of the lateral parabrachial nucleus (LPBel), which, in turn, project principally to the median preoptic subnucleus (MnPO) of the preoptic area (POA) (Bratincsak and Palkovits, 2004; Nakamura and Morrison, 2008). In parallel, glutamatergic excitation of POA-projecting neurons in the dorsal subnucleus of the LPB (LPBd) (Bratincsak and Palkovits, 2004; Nakamura and Morrison, 2010) is necessary for the skin warming-evoked inhibition of BAT thermogenesis (Nakamura and Morrison, 2010). Thus, both cool and warm cutaneous thermosensory signals, transmitted from the spinal dorsal horn or trigeminal neurons to the POA by separate populations of LPB neurons (Fig. 1) are essential for eliciting the rapid increases and decreases, respectively, in BAT SNA and BAT thermogenesis that contribute to the defense of TCORE during environmental thermal challenges (Nakamura and Morrison, 2008).
Viscerosensory afferents, with axons in the vagus nerve and synapsing on second-order neurons in the nucleus of the solitary tract (NTS), can influence BAT activity (Szekely, 2000) (Figs. 2D, 2E). For instance, the inhibition of BAT activity induced by upregulation of hepatic glucokinase (Tsukita et al., 2012) and the BAT activation following either intragastric delivery of the TRP agonist, capsiate (Ono et al., 2011) or the presence of lipids in the duodenum (Blouet and Schwartz, 2012) are mediated by vagal afferents. Arterial chemoreceptor afferents synapsing in the commissural NTS (commNTS) and signaling systemic hypoxia, elicit a marked inhibition of BAT SNA and BAT thermogenesis (Madden and Morrison, 2005) to restrict oxygen consumption in the face of reduced oxygen availability. The NTS also receives inputs from brainstem and forebrain sites involved in metabolic regulation and these provide the additional potential for NTS neurons to integrate a variety of metabolic signals influencing BAT thermogenesis.
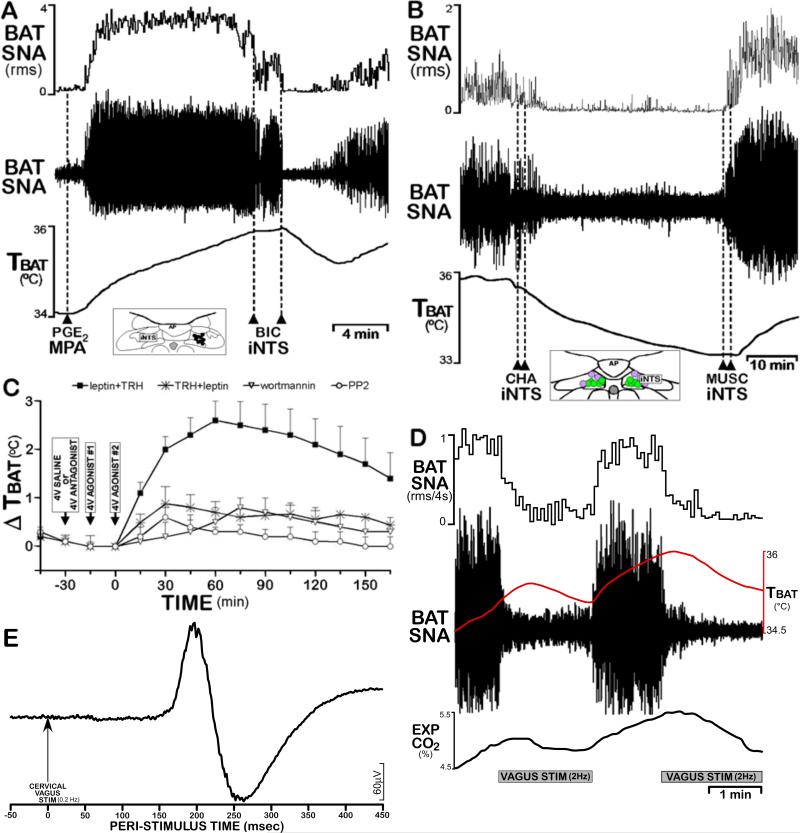
Figure 2. NTS neurons mediate both inhibition and excitation of BAT thermogenesis.
(A) Disinhibitory activation of neurons in intermediate NTS (iNTS) at the level of the area postrema (insert) with injections of bicuculline (BIC) completely reversed the PGE2 in medial preoptic area (MPA)-mediated increases in BAT SNA and BAT temperature (TBAT). Modified from (Cao et al., 2010).
(B) Activation of adenosine 1A receptors in iNTS with injections of the agonist, N6-cyclohexyladenosine (CHA), reverses the cooling-evoked increases in BAT SNA and TBAT. Inhibition of iNTS neurons with injections of muscimol (MUSC) reverses the CHA-evoked inhibition of BAT SNA, consistent with adenosine producing an increase in the activity of BAT sympathoinhibitory neurons in iNTS. Modified from (Tupone et al., 2013).
(C) Changes in TBAT elicited by agents applied to the 4th ventricle. When preceded by leptin, TRH markedly increases TBAT, indicating BAT thermogenesis. This effect is prevented by prior blockade of signal transduction pathways via 4th ventricle administration of wortmannin to block leptin-evoked PIP3 generation or by the Src-SH2 antagonist, PP2. Modified from (Rogers et al., 2009).
(D) Stimulation (400μA, 1 ms pulses, 2 Hz) of afferents in the cervical vagus elicits a potent inhibition of cooling-evoked increases in BAT SNA, TBAT and expired CO2, an indicator of metabolic oxygen consumption. These data reflect a vagal afferent drive to BAT sympathoinhibitory NTS neurons. Author's unpublished observation.
(E) Paired shock stimulation (400μA, 1 ms pulses, 6 ms interpulse interval, 0.2 Hz) of afferents in the cervical vagus nerve evokes an excitatory compound action potential in BAT SNA, consistent with a population of vagal afferents activating BAT sympathoexcitatory neurons in NTS. Author's unpublished observation.
The NTS contains BAT sympathoinhibitory neurons. The bicuculline-evoked blockade of GABAA receptors in the intermediate NTS (iNTS) potently inhibits cooling-evoked and febrile increases in BAT SNA (Cao et al., 2010) (Fig. 2A) and the inhibition of BAT SNA following injection of an adenosine A1 receptor agonist into iNTS is dependent on the activity of iNTS neurons (Tupone et al., 2013) (Fig. 2B). As byproducts of ATP metabolism, adenosine and adenosine 5’-monophosphate, whose central administration also produces hypothermia (Muzzi et al., 2012), can diffuse from cells and may function to reduce energy consumption in situations (e.g., hypoxia or caloric restriction) of reduced energy substrate availability. A dramatic fall in overall metabolism, including an inhibition of BAT thermogenesis (Cannon and Nedergaard, 2004), coupled with the resulting hypothermia, is a hallmark of the torpid response to reduced fuel availability. Establishing such a hypometabolic state in non-hibernators, either through an adenosine A1 receptor-mediated activation of the BAT sympathoinhibitory pathway from the NTS (Tupone et al., 2013), or via inhibition of thermogenic premotor neurons in the rostral raphe pallidus (rRPa) (Cerri et al., 2013) could provide the benefits of therapeutic hypothermia for recovery from ischemic stroke or cardiac arrest. NTS may also contain neurons that produce BAT activation (Fig. 2E). In this regard, leptin and thyrotropin–releasing hormone (TRH) (Rogers et al., 2009) (Fig. 2C) and the melanocortin 3/4 receptor (MC3/4-R) agonist, melanotan II (MTII), (Williams et al., 2003) activate BAT when applied to the 4th ventricle, potentially via stimulation of neurons in the NTS.
HYPOTHALAMIC INFLUENCES ON BAT THERMOGENESIS
As the central integrator of many dimensions of the homeostatic space, the hypothalamus occupies a pivotal position between the sensation of skin and core temperatures and the sympathetic premotor pathways controlling BAT thermogenesis. Additionally, the febrile stimulation of BAT thermogenesis is mediated by the action of prostaglandin (PG) E2 on its EP3 receptors on hypothalamic neurons. Thermoregulatory control of BAT thermogenesis and BAT energy expenditure is but one of the myriad of interrelated homeostatic functions embedded in the hypothalamic matrix, thus, the latter provides a rich substrate for non-thermal influences on BAT energy expenditure.
The POA, including the MnPO and the medial preoptic area (MPA), contains GABAergic (Lundius et al., 2010), intrinsically warm-sensitive neurons whose activity increases with local hypothalamic temperature and is inhibited in response to skin cooling. Since sympathetic BAT thermogenesis is augmented by skin cooling (Nakamura and Morrison, 2007) or direct cooling of the POA, by brain transections immediately caudal to the POA, and by local inhibition of POA neuronal activity (Yoshida et al., 2009), warm-sensitive POA neurons are postulated to integrate cutaneous and core thermal signals and to provide a GABAergic inhibitory input to BAT sympathoexcitatory neurons in the dorsomedial hypothalamus/dorsal hypothalamic area (DMH/DHA) and/or the rRPa (Dimitrov et al., 2011; Nakamura et al., 2009; Yoshida et al., 2009). The MnPO also contains glutamatergic neurons that project to the DMH/DHA, that are synaptically connected to BAT, and that receive glutamatergic terminals containing tuberoinfundibular peptide of 39 residues (TIP39), which produces an increase in core temperature when injected into the MnPO (Dimitrov et al., 2011). MnPO neurons expressing the leptin receptor (LepRb) also project to DMH/DA (Zhang et al., 2011a). Thus, glutamatergic inputs to DMH/DHA (Madden and Morrison, 2004) could provide the excitation required to drive the BAT sympathoexcitatory neurons in DMH/DHA when their POA inhibitory input is reduced during skin cooling or fever. The strong activation of BAT thermogenesis by local nanoinjections of bicuculline into MnPO could arise from a disinhibition of either (or both) the skin cooling-activated inhibitory interneurons in MnPO or a MnPO glutamatergic input to DMH/DHA.
Although not yet directly confirmed in human, augmented BAT thermogenesis contributes to the febrile elevation in rodent TCORE during the acute phase reaction to endogenous pyrogens released during infection or inflammation (Nakamura et al., 2002) and inhibition of BAT thermogenesis contributes to the hypothermic response to elevated bacterial LPS, as in sepsis and endotoxic shock. Central delivery of the inflammatory cytokine, interleukin 1β, or tumor necrosis factor alpha (Arruda et al., 2010) potently activated BAT thermogenesis, which is also augmented in cachexia. PGE2, synthesized in the brain vasculature and in peripheral tissues, is a powerful endogenous pyrogenic mediator that binds to EP3 receptors in the POA (Lazarus et al., 2007; Nakamura et al., 2002) and produces an activation of BAT thermogenesis (Madden and Morrison, 2003; Nakamura et al., 2002) by inhibiting the POA warm-sensitive, GABAergic inhibition (Lundius et al., 2010; Nakamura et al., 2002; Yoshida et al., 2003) of BAT sympathoexcitatory neurons in DMH/DHA (Nakamura et al., 2002; Nakamura et al., 2005) and potentially in the rRPa (Nakamura et al., 2004; Nakamura et al., 2009). Potential sources of the glutamatergic drive to DMH/DA neurons supporting the febrile activation of BAT thermogenesis include glutamatergic neurons in the MnPO (Dimitrov et al., 2011) and orexin/glutamatergic neurons in perifornical lateral hypothalamus (PeF/LH) (Takahashi et al., 2013).
Central histamine administration activates BAT and increases TCORE, potentially acting in the paraventricular hypothalamus (PVH) or the POA (Lundius et al., 2010; Yasuda et al., 2004). Both a histamine1 receptor (H1-R)-evoked depolarization of local non-GABAergic (potentially glutamatergic, BAT sympathoexcitatory) neurons and a histamine3 receptor (H3-R)-mediated inhibition of GABAergic (potentially warm-sensitive, BAT sympathoinhibitory) neurons (Lundius et al., 2010; Tabarean, 2012) could contribute to the POA histaminergic stimulation of BAT. The reduced POA H1-R-induced stimulation of BAT UCP1 in diet induced obese (DIO) mice and the reduced BAT energy expenditure accompanying the antagonism of hypothalamic H1-R by antipsychotic drugs (He et al., 2013) could contribute to weight gain in these models of obesity. Alpha1 adrenergic receptor agonists inhibit BAT thermogenesis and decrease TCORE when injected into the MnPO (Osaka, 2009). Stimulation of central D2 dopamine receptors inhibits BAT SNA and BAT thermogenesis via an unknown mechanism.
The DMH/DHA contains BAT sympathoexcitatory neurons (Cao et al., 2004) that are synaptically-connected to BAT (Bamshad et al., 1999; Cano et al., 2003; Oldfield et al., 2002; Zhang et al., 2011a) via their projection to BAT sympathetic premotor neurons in the rRPa (Nakamura et al., 2005; Yoshida et al., 2009; Zhang et al., 2011a). During cold-defense and fever, when their tonic GABAergic inhibition (Cao et al., 2004) from the POA (Nakamura et al., 2005) (Fig. 1) is reduced, glutamatergic inputs to BAT sympathoexcitatory neurons in the DMH/DA, including many expressing LepRb, provide the synaptic drive essential for stimulation of BAT SNA and BAT thermogenesis (Cano et al., 2003; Madden and Morrison, 2004; Nakamura and Morrison, 2007; Nakamura et al., 2005; Rathner and Morrison, 2006; Sarkar et al., 2007; Yoshida et al., 2009; Zhang et al., 2011a). The excitatory inputs to DMH/DHA could include the MnPO (Dimitrov et al., 2011); the PeF/LH (Takahashi et al., 2013) and the periaqueductal gray (PAG) (de Menezes et al., 2009). A role for the melanocortin 4 receptor (MC4-R) in the DMH in the regulation of BAT is supported by increased BAT UCP-1 mRNA after injection of the MC4-R agonist, MTII, in DMH and attenuation of systemic MTII-evoked increases in TBAT by injection of an MC4-R antagonist in DMH (Enriori et al., 2011).
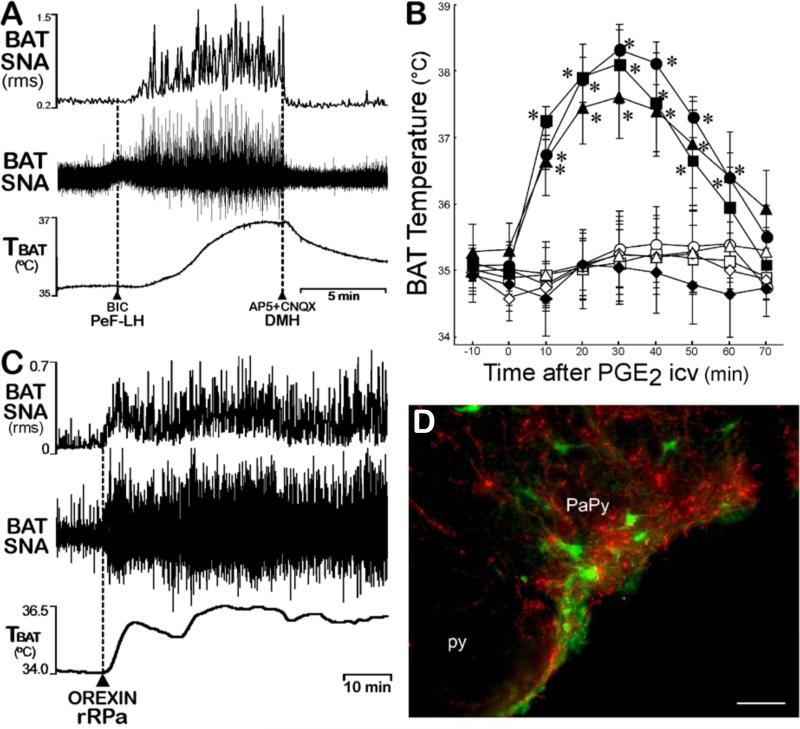
A subpopulation of the orexinergic/glutamatergic neurons in the PeF/LH projects to BAT sympathetic premotor neurons in the rRPa and parapyramidal (PaPy) regions of the rostral ventromedial medulla (Berthoud et al., 2005; Oldfield et al., 2002; Tupone et al., 2011) (Fig. 3D). Central orexin induces c-fos in rRPa neurons (Berthoud et al., 2005) and activation of PeF-LH neurons or nanoinjection of orexin specifically into the rRPa increases BAT activity (Tupone et al., 2011) (Fig. 3C). Orexin potentiated an ongoing thermoregulatory activation of BAT sympathetic premotor neurons in the rRPa (Fig. 3C), but had a minimal effect on the low level of BAT SNA under thermoneutral conditions (Tupone et al., 2011), consistent with an orexinergic amplification of excitatory inputs to BAT sympathetic premotor neurons in the rRPa that regulate BAT thermogenesis. The finding that cold-defensive, febrile and stress-induced thermogenesis are eliminated in orexin neuron-ablated, but not in orexin-KO mice (Takahashi et al., 2013) (Fig. 3B), suggests that glutamate release from PeF-LH orexin neurons may also have a potent excitatory influence on BAT activation, although the critical site of such glutamate release from PeF/LH axons remains unknown. These data are confounded by the failure of an LH injection of the GABAA agonist, muscimol, to reduce PGE2-evoked increases in BAT SNA (Nakamura et al., 2005), although disinhibition of PeF-LH neurons elicits a potent activation of BAT SNA that is reversed by glutamate receptor antagonists in DMH/DA (Cerri and Morrison, 2005) (Fig. 3A). Reduced functionality of the orexin system, as in narcolepsy, is accompanied by disruptions in thermoregulation and in metabolism, with a propensity for obesity and metabolic syndrome (Plazzi et al., 2011), to which reduced BAT activity may contribute.
Figure 3. Orexinergic and other PeF/LH neurons influence BAT thermogenesis.
(A) The activation of BAT SNA and BAT thermogenesis produced by disinhibitory activation of LH neurons with bicuculline (BIC) nanoinjection is dependent on the activation of glutamate receptors on DMH/DA neurons. Modified from (Cerri and Morrison, 2005).
(B) Icv PGE2 (filled symbols), but not ACSF (open symbols), elicited a marked increase in BAT temperature in orexin-KO mice (triangles) and in the wild-type littermates for orexin-KO (circles) and for orexin neuron-ablated (squares), but had no effect on TBAT in orexin neuron-ablated mice (diamonds). Modified from (Takahashi et al., 2013).
(C) Under cool conditions (TCORE < 37 °C) with a low level of basal BAT SNA, nanoinjection of orexin-A (dashed line) in the rRPa elicited a prolonged increase in BAT SNA and TBAT. Modified from (Tupone et al., 2011).
(D) Orexinergic fibers (red) surround putative BAT sympathetic premotor neurons in PaPy (and in rRPa) transynaptically-infected following PRV inoculations of interscapular BAT. Modified from (Tupone et al., 2011).
Leptin acts through LepRb to enhance the excitability of neurons in the pathways controlling BAT activity and this permissive effect, perhaps indicating the adequacy of stored and incoming fuel supplies, is necessary for the activation of BAT thermogenesis. Administration of leptin to leptin-deficient, ob/ob mice increases BAT UCP-1 mRNA and protein. In fasted rats with low leptin levels, but not in fed rats, intracerebroventricular (icv) leptin increased BAT activity. However, as might be expected if leptin is providing a permissive signal, ad libitum fed animals, particularly in a thermoneutral environment, may have little BAT response to exogenous leptin (Sivitz et al., 1999), although supraphysiological levels of leptin have been effective in other studies on fed animals (Haynes et al., 1997; Morrison, 2004). High fat fed mice had impaired BAT SNA responses to systemic leptin, although diet induced obese mice retained the increase in TBAT evoked by direct intraparenchymal injection of leptin (Enriori et al., 2011).
Of the several central sites containing LepRb-expressing neurons (Zhang et al., 2011a) and implicated in the regulation of BAT, including the arcuate nucleus (ARC), DMH, MnPO, and NTS, neurons in the ARC have been the focus for the leptin effect on BAT activation. Leptin injection in ARC increases BAT SNA and deletion of ARC leptin receptors prevented leptin-stimulated BAT SNA (Harlan et al., 2011). Leptin may activate GABAergic, RIP-Cre neurons in the ARC that project to the PVH to inhibit a glutamatergic drive to NTS GABAergic neurons that innervate the RPa (Kong et al., 2012), although a projection from the NTS to the rRPa was not confirmed (Tupone et al., 2013). Leptin injection into DMH also increases TBAT (Enriori et al., 2011), although the injection volumes were large and anatomical control injections were not performed. Finally, leptin when co-administered with TRH into the 4th ventricle increases TBAT (Rogers et al., 2009) likely via effects within the NTS.
MTII activation of central MC3/4-R, the receptor for α-melanocyte-stimulating hormone (α-MSH) produced by proopiomelanocortin neurons in the ARC, increases BAT SNA (Haynes et al., 1999) and chronic blockade of MC3/4-R with icv SHU9119 decreases TBAT and BAT UCP-1 mRNA (Verty et al., 2010). In contrast, indirect calorimetry indicates an increased energy expenditure in MC4-R KO mice when appropriately expressed on a ‘per mouse’ basis or normalized to lean mass (Butler and Kozak, 2010) and obese MC4-R KO mice have increased TBAT compared with their littermates (Enriori et al., 2011). These conflicting observations remain unresolved. MC4-R are expressed in many neurons synaptically connected to BAT including those in the PVH, where MTII activates BAT (Song et al., 2008), the sub zona incerta (subZI), the DMH, the ventrolateral medulla (VLM) and the raphe (Song et al., 2008). MC4-R agonists in the subZI increased, and MC4-R antagonism decreased, TBAT, consistent with an endogenous activation of subZI MC4-R contributing to BAT thermogenesis. In MC4-R transcription-blocked mice, re-expression of MC4-R selectively in the PVH and amygdala Sim1-Cre neurons did not rescue the absence of MTII-induced oxygen consumption, suggesting that the energy expenditure stimulating effects of MC4-R activation are not mediated by PVH and/or amygdala MC4-R (Balthasar et al., 2005). However, it is important to note that re-expression of MC4-R in Sim1-expressing neurons would not rescue the expression of MC4-R on presynaptic terminals in PVH and activation of presynaptic MC4-R could play a role in the regulation of BAT (Fig. 4E).
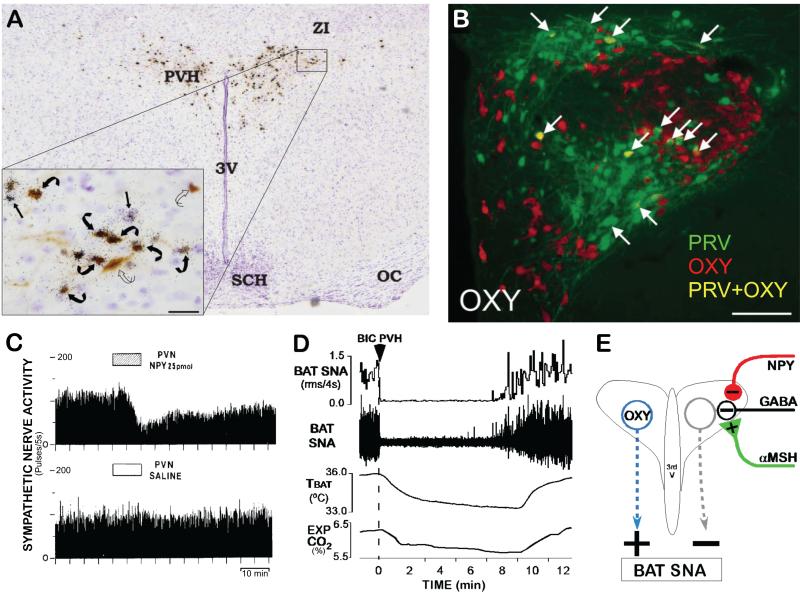
Figure 4. Paraventricular hypothalamic nucleus (PVH) mechanisms influencing BAT thermogenesis.
(A) Histological section through the PVH illustrating the overlap of transynaptically infected, PRV-labeled neurons (brown) following PRV injections into interscapular BAT and in situ hybridization for melanocortin 4-receptor (MC4-R) mRNA expression (black granules). Inset: High magnification of the outlined portion of the PVH. Note the presence of PRV in neurons surrounded by MC4-R (curved black arrows) and PRV in neurons without associated MC4-R (curved open arrows). Bar = 25mm. Modified from (Song et al., 2008).
(B) Immunolabeling of PVH neurons for transynaptic infection with PRV (green) after PRV injections into interscapular BAT and for oxytocin (OXY; red). Arrows indicate neurons containing both PRV and OXY (yellow). Modified from (Oldfield et al., 2002).
(C) Microinjection of neuropeptide Y (NPY), but not saline vehicle, into the PVH inhibited BAT SNA. Modified from (Egawa et al., 1991).
(D) Nanoinjection of bicuculline (BIC) into the PVH completely reversed the cooling-evoked increases in BAT SNA, BAT temperature (TBAT) and expired CO2 (Exp CO2). Modified from (Madden and Morrison, 2009).
(E) Schematic of the proposed neurocircuitry mediating influences on BAT thermogenesis mediated by PVH neurons and their GABAergic regulation. Based in part on (Cowley et al., 1999).
Whether an α-MSH activation of MC4-R plays a role in the leptin-induced stimulation BAT thermogenesis remains controversial. Leptin failed to increase UCP-1 mRNA in MC4-R KO mice (Ste Marie et al., 2000) and blockade of MC4-R prevents leptin-evoked increases in BAT UCP-1. However, leptin increases TBAT in MC4-R KO mice (Enriori et al., 2011) and the leptin-evoked increase in BAT SNA is not blocked by the MC3/4-R antagonist, SHU9119, even in doses that prevent the MC4-R agonist-induced activation of BAT SNA (Haynes et al., 1999). In the case of pituitary adenylate cyclase-activating polypeptide (PACAP), whose elimination produces cold-intolerance due to reduced sympathetically-mediated BAT thermogenesis, the increased BAT SNA following central PACAP is dependent on MC3/4-R activation.
Neurons in the PVH are pauci-synaptically connected to BAT (Bamshad et al., 1999; Cano et al., 2003; Oldfield et al., 2002; Yoshida et al., 2003) (Fig. 4A). The PVH contains BAT sympathoinhibitory neurons since activation of neurons in PVH completely reverses cooling-evoked and febrile increases in BAT SNA (Fig. 4D) (Madden and Morrison, 2009). Presynaptic regulation of transmitter release by peptidergic neuromodulators strongly influences the level of BAT sympathoinhibition from PVH. PVH administration of neuropeptide Y (NPY) increases the activity of PVH neurons by presynaptically inhibiting local GABA release (Cowley et al., 1999) (Fig. 4E) and decreases BAT activity (Fig. 4C) (Egawa et al., 1991). Tonic release of NPY also has a sympathoinhibitory effect on the central regulation of BAT thermogenesis, since NPY KO mice have increased energy expenditure (increased O2 consumption), increased BAT UCP-1 during fasting, and are less susceptible to DIO (Patel et al., 2006). The NPY inputs to the PVH come from the ARC, the DMH, and the VLM. Consistent with an NPY input from DMH augmenting the BAT sympathoinhibitory effect of PVH neurons, NPY neurons in DMH project to PVH, but not to rRPa or NTS (Lee et al., 2013) and knockdown of NPY in the compact DMH increased BAT UCP-1 expression and cold-evoked thermogenesis and stimulated “browning” in inguinal white adipose tissue (Chao et al., 2011). In contrast, activation of MC4-R, which are located in the vicinity of PVH neurons synaptically-connected to BAT (Song et al., 2008) (Fig. 4A) and which presynaptically potentiate GABA release onto PVH neurons (Cowley et al., 1999) (Fig. 4E), increases TBAT (Skibicka and Grill, 2009; Song et al., 2008). Although a selective rescue of MC4-R expression in the PVH neurons of MC4-R−/− mice failed to elevate their oxygen consumption to wild-type levels (Balthasar et al., 2005), this approach would only rescue postsynaptic MC4-R in PVH, but not those located presynaptically and potentially responsible for the effects on BAT activity of melanocortin receptor ligands in PVH. The physiological stimuli activating the BAT sympathoinhibitory output from the PVH are unknown, but may include hypoglycemia and chronic intermittent hypoxia. If PVH neurons provide a tonic inhibition of BAT thermogenesis, release from this inhibition under specific conditions, such as changes in dietary composition, could chronically alter the level of BAT activation and BAT energy expenditure. For example, the BAT stimulating effects of leptin in the ARC have been attributed to LepRb-mediated activation of PVH-projecting, GABAergic RIP-Cre neurons that reduce the activity of tonically-active, BAT sympathoinhibitory neurons in the PVH (Kong et al., 2012). However, naltrexone into rostral NTS blocked the feeding response to NPY in the PVH, but not the BAT inhibitory effect.
A role for oxytocinergic PVH projection neurons in the regulation of BAT thermogenesis is supported by the anatomical finding that oxytocin neurons in the caudal PVH are transynaptically infected following pseudorabies virus (PRV) injection into BAT (Oldfield et al., 2002) (Fig. 4B) and by indirect evidence that oxytocinergic neurotransmission contributes an excitatory drive to BAT sympathetic outflow. Cold defensive thermoregulation is impaired in oxytocin- and oxytocin receptor-deficient mice and cold defense is recovered by restoring oxytocin receptors in the hypothalamus (Goke et al., 1995). A reduction of PVH oxytocin by 50% through Sim1 neuron ablation reduced TBAT and TCORE and resting energy expenditure (Xi et al., 2012). Ablation of oxytocin neurons reduced high fat diet-induced energy expenditure (Wu et al., 2012), consistent with a role for oxytocin neurons in diet-induced activation of BAT. Similarly, obese patients with Prader-Willi syndrome have decreased numbers of oxytocin neurons, suggesting that a reduced BAT energy expenditure could contribute to their weight gain. Central administration of corticotropin releasing hormone (CRF) activates BAT (Cerri and Morrison, 2006; Correia et al., 2001; Egawa et al., 1990), potentially through CRF receptors in the MPA or in the DMH/DA, but not those in PVH (Cerri and Morrison, 2006; Egawa et al., 1990). CRF receptor activation contributes to the BAT sympathetic response to systemic leptin and to icv interleukin 1β.
Other neurotransmitters influence BAT thermogenesis by altering the activity of neurons in PVH. 5-hydroxytryptamine (5-HT) in the PVH increases BAT SNA. NE in the PVH causes a biphasic response (inhibition followed by excitation) in BAT SNA and NPY released from central catecholaminergic neurons may activate BAT, since overexpression of NPY in dopamine-β-hydroxylase (DBH)-expressing neurons increased guanosine diphosphate (GDP) binding in BAT mitochondria. Neurotrophins may influence energy homeostasis via PVH, e.g., brain-derived neurotrophic factor (BDNF) into the PVH increased BAT UCP-1 and decreased body weight (Wang et al., 2007). Further, enriched environments lead to increased ‘browning’ of retroperitoneal WAT via activation of its sympathetic input, an effect mimicked by overexpression of BDNF in the hypothalamus and blocked by inhibition of hypothalamic BDNF signaling (Cao et al., 2011).
Indirect evidence suggests that neurons in the ventromedial hypothalamus (VMH) could contribute to activation of BAT, although the pathways through which they might exert an influence on BAT sympathetic premotor neurons in rRPa remain unidentified. Electrical stimulation of the VMH increased BAT thermogenesis (Perkins et al., 1981) and microinjection of glutamate or thyroid hormone, triiodothyronine (T3), (Lopez et al., 2010) into VMH increases BAT SNA. Conversely, electrolytic lesions of the VMH attenuated BAT SNA (Niijima et al., 1984) and mice lacking phosphoinositide 3-kinase (PI3K) specifically in the steroidogenic factor-1 (SF-1)-containing neurons of the VMH had impaired diet-induced thermogenesis and lower UCP-1 in BAT (Klockener et al., 2011). However, the electrical stimulation and lesioning and the large injection volumes without anatomical controls in these studies, and the absence transynaptic infection of VMH neurons following PRV inoculations of BAT (Bamshad et al., 1999; Cano et al., 2003; Oldfield et al., 2002) limit conclusions on the role of VMH neurons in the control of BAT thermogenesis.
Icv delivery of T3, or localized injection into the VMH of either T3 or a dominant-negative 5' adenosine monophosphate-activated protein kinase (AMPK)α increased BAT SNA and the expression of thermogenic markers in the BAT of euthyroid rats (Lopez et al., 2010). This stimulation of BAT activity is postulated to arise from a thyroid hormone receptor driven reduction in AMPK, allowing increased lipid synthesis in VMH neurons and an elevation in their discharge rate (Lopez et al., 2010), although the mechanism through which increased lipid synthesis increases neuronal discharge, as well as the pathway linking the VMH to BAT sympathetic premotor neurons in the rRPa remain unknown. Inconsistent with these results, icv injection of a fatty acid synthase inhibitor elicits a strong and rapid increase in TBAT. Systemic or icv injection of TRH, as well as local injections into the DMH, POA, and VMH increase BAT activation (Griffiths et al., 1988). Delivery of TRH into the 4th ventricle, where it would potentially reach the NTS, increased TBAT, an effect that was enhanced by prior application of leptin to the ventricle (Rogers et al., 2009) (Fig. 2C). TRH-expressing neurons in the RPa are activated by cold-exposure (Cabral et al., 2012), but their role in regulating BAT thermogenesis is unknown.
Bone morphogenic protein 7 (BMP7) promotes differentiation of brown pre-adipocytes, increases BAT energy expenditure and thermogenesis and reduces weight gain (Tseng et al., 2008), results suggesting that BMP7 supports BAT activation via both peripheral and central mechanisms. Central BMP8B increases BAT SNA and BAT thermogenesis (Whittle et al., 2012). The CNS pathways mediating the BMP stimulations of BAT activity are unknown.
Icv angiotensin II (AngII) decreases body weight gain in part via activation of BAT. Genetically-driven hyperactivity of the neuronal renin-angiotensin system increased BAT SNA and TCORE, but not BAT UCP-1 mRNA (Grobe et al., 2010), consistent with activation of the central renin-angiotensin system activating BAT and also providing another example where the activity of BAT is dissociated from upregulation of BAT UCP-1 (Nedergaard and Cannon, 2013). Curiously, in mice with genetically-driven neuronal hyperactivity of the renin-angiotensin system, the BAT SNA is not temperature responsive (Grobe et al., 2010). Conversely, leptin-evoked increases in BAT SNA were prevented by icv losartan, an angiotensin1 (AT1) receptor antagonist, and impaired in AT1A receptor KO mice (Hilzendeger et al., 2012). AT1A receptor deletion from PVH neurons leads to impaired energy expenditure on a high fat diet resulting in increased weight gain (de Kloet et al., 2013), suggesting that the PVH is a site at which angiotensin may increase BAT metabolism.
Forebrain cannabinoid type 1 receptor (CB1-R) stimulation mediates a tonic inhibitory effect on sympathetically-mediated BAT thermogenesis, although the relevant site of the CB1-R and the source(s) of the endogenous CB1-R agonist are unknown. Deletion of CB1-R in the hypothalamus (Cardinal et al., 2012), or forebrain overexpression of the endocannabinoid-inactivating, monoacylglycerol lipase (Jung et al., 2012) or chronic CB1-R antagonism (Bajzer et al., 2011) increase BAT activation and overall energy expenditure. The elevated BAT thermogenesis following deletion of CB1-R in the forebrain, NTS and some sympathetic ganglion cells contributes to a resistance to diet-induced obesity (Quarta et al., 2010). CB1-R activation is necessary for the inhibition of BAT thermogenesis that contributes to the hypothermic response to bacterial lipopolysaccharide (LPS) (Steiner et al., 2011).
Central insulin can exert a stimulatory or inhibitory influence on BAT thermogenesis, depending on the dose and site of administration and the glucose status, but the experimental insulin doses have not been compared to physiological extracellular levels of brain insulin. Central administration of high doses of insulin increased BAT SNA (Rahmouni et al., 2004) and BAT GDP binding in fed, but not in food-restricted rats. Local injections of insulin into the POA (but not the DMH or rRPa) increased BAT activity, potentially by inhibiting POA warm-sensitive neurons (Sanchez-Alavez et al., 2010). Central administration of low doses of insulin, or direct injections into VMH or PVH, decreased BAT SNA, but co-administration of insulin and glucose stimulated BAT SNA. Streptozotocin-induced diabetic rats are cold intolerant due to a reduced capacity for central activation of BAT thermogenesis (Rothwell and Stock, 1981). Further, the magnitude of the depression of BAT activity evoked by insulin in the VMH had a diurnal sensitivity, while the reduction in BAT activity following insulin into the suprachiasmatic nucleus observed in the light period was reversed to an increase during the dark phase (Sakaguchi et al., 1988b).
Central delivery of the pancreatic hormone, amylin, elevated BAT SNA and increased TCORE (Fernandes-Santos et al., 2013), although the site of amylin action remains undetermined. Overexpression of receptor activity-modifying protein1, involved in the G protein-coupled receptor binding of amylin, also increased BAT SNA and TCORE (Zhang et al., 2011b).
Administration of glucagon increases BAT thermogenesis, at least partly via a central mechanism since icv injections of glucagon, glucagon-like peptide-1 (GLP-1), or oxyntomodulin, a dual GLP-1 receptor and glucagon receptor agonist, increase BAT SNA and TBAT (Lockie et al., 2012). Although the mRNA, peptide and binding site distributions for pre-pro-glucagon and GLP-1 in the CNS are documented, the site(s) of action of central glucagon or glucagon-like peptides to activate BAT are not known.
BRAINSTEM EFFERENT REGULATION OF BAT THERMOGENESIS
Within the hierarchical organization of the central network regulating BAT SNA and BAT thermogenesis, neurons in the rostral ventromedial medulla, centered in the rRPa and extending into the nearby raphe magnus nucleus and over the pyramids to the PaPy (Bamshad et al., 1999; Cano et al., 2003; Oldfield et al., 2002; Yoshida et al., 2003), are the principal BAT sympathetic premotor neurons – providing the essential excitatory drive to BAT sympathetic preganglionic neurons (SPNs) in the intermediolateral nucleus (IML) of the thoracolumbar spinal cord, which, in turn, excite sympathetic ganglion cells innervating the BAT pads (Fig. 1). Inhibition of rRPa neuronal activity produces dramatic falls in TCORE in conscious rats (Zaretsky et al., 2003) and reverses the increases in BAT SNA elicited by a variety of thermogenic stimuli, including central and systemic PGE2 (Nakamura et al., 2002), skin cooling (Nakamura and Morrison, 2007), disinhibition of DMH/DA (Cao et al., 2004) or lateral hypothalamic (Cerri and Morrison, 2005) neurons, activations of central mu-opioid receptors, central melanocortin receptors, preoptic CRF receptors (Cerri and Morrison, 2006) and systemic administration of the adipose tissue hormone, leptin.
Both glutamatergic (i.e., vesicular glutamate transporter 3 (VGLUT3)-expressing) and serotonergic neurons in the rRPa region are anatomically and functionally related to the activation of BAT thermogenesis (Cano et al., 2003; Martin-Cora et al., 2000; Nakamura et al., 2004). Under thermoneutral or other conditions with low BAT SNA, the discharge of BAT sympathetic premotor neurons in the rRPa is dominated by a GABAergic inhibition (Morrison et al., 1999), which is overcome by a potent glutamatergic excitation, particularly that from the DMH (Cao and Morrison, 2006), during the cold-evoked and febrile activations of BAT (Madden and Morrison, 2003; Nakamura and Morrison, 2007). BAT sympathetic premotor neurons in rRPa are excited by local application of agonists for NMDA and non-NMDA subtypes of glutamate receptors. In contrast, systemic administration of the 5-HT1A inhibitory receptor agonist, 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), decreases TBAT, likely via activation of 5-HT1A receptors in the rRPa, where direct injection of 8-OH-DPAT inhibits BAT SNA (Morrison, 2004; Nakamura and Morrison, 2007), likely through inhibition of serotonergic and non-serotonergic BAT sympathetic premotor neurons. MTII in the medullary raphe increases TBAT, although the phenotype of the relevant rRPa neurons is unknown.
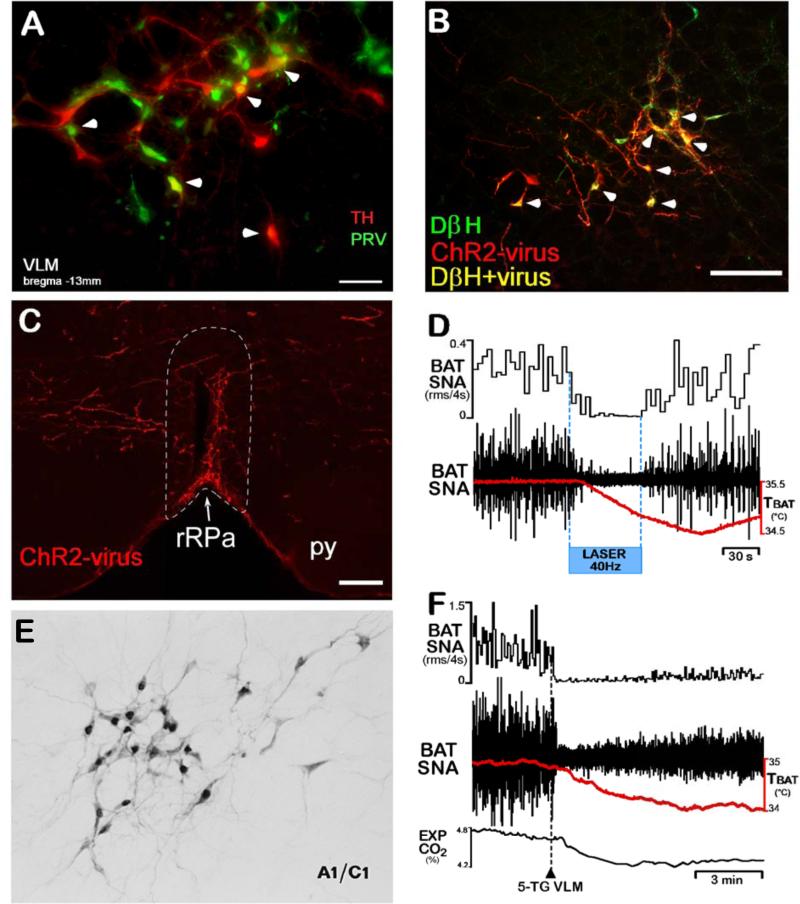
Neurons in the VLM, including catecholaminergic neurons, are transynaptically infected following PRV injections into BAT (Cano et al., 2003; Madden et al., 2013) (Fig. 5A). Activation of NMDA receptors or blockade of GABAA receptors or laser light activation of ChR2-transfected neurons in the VLM region corresponding to the locations of the A1 and C1 catecholaminergic cell groups elicits a prompt and complete inhibition of the cooling-evoked or febrile increases in BAT SNA and BAT thermogenesis (Cao et al., 2010; Madden et al., 2013) (Fig. 5D). These observations likely reflect multiple BAT sympathoinhibitory systems within the rostrocaudal extent of the VLM, since the intermediate and caudal VLM contain neurons that project directly to rRPa (Figs. 5B, 5C), while the RVLM does not (Madden et al., 2013). One component of the BAT sympathoinhibitory system in the intermediate VLM is a direct projection of catecholaminergic neurons to the rRPa (Figs. 5B, 5C), where activation of α2 adrenergic receptors inhibits BAT sympathetic premotor neurons and blocks cold-evoked and febrile stimulations of BAT thermogenesis (Madden et al., 2013) (Fig. 5D), likely accounting for the hypothermic response to systemic administration of α2 adrenergic receptor agonists. Although the specific physiological stimuli that drive the catecholaminergic inhibition of BAT activity remain undefined, potential roles in hypoxia or glucoprivation-induced inhibition of BAT are suggested by the observations that these stimuli activate catecholaminergic neurons of the VLM (Ritter et al., 1998) (Fig. 5E) and inhibit BAT SNA and BAT thermogenesis (Madden, 2012; Madden and Morrison, 2005) (Fig. 5F), consistent with VLM neurons contributing to a restricted BAT energy consumption in the face of a reduction in metabolic fuel availability.
Figure 5. Neurons in the ventrolateral medulla (VLM), including catecholaminergic neurons, regulate BAT SNA and BAT thermogenesis.
(A) The A1/C1 area of the VLM (bregma −13 mm) contains transynaptically-infected neurons (green) following PRV injections into interscapular BAT, tyrosine hydroxylase (TH)-immunoreactive neurons (red), and double-labeled (yellow, arrowhead) neurons.
(B) Following PRSx8-channel rhodopsin 2-mCherry (ChR2) lentivirus nanoinjections into VLM, many dopamine beta hydroxylase (DβH)-expressing neurons (green) are double-labeled (yellow, arrowheads) due to transfection with ChR2 (red).
(C) Following PRSx8-channel rhodopsin 2-mCherry (ChR2) lentivirus nanoinjections into VLM, the rRPa (white dotted outline) contains highly varicose fibers (red) expressing ChR2.
(D) Laser photostimulation of VLM neurons containing the ChR2 (largely catecholaminergic neurons) inhibited BAT SNA and reduced TBAT, an effect that was attenuated by blockade of α2-adrenergic receptors in the rRPa. Panels A – D, suggesting that activation of catecholaminergic neurons in the VLM inhibits BAT SNA via direct catecholaminergic inputs to the rostral raphe pallidus (rRPa), are modified from (Madden et al., 2013).
(E) Glucoprivation (2-DG, iv) activates (increases c-fos (black nuclei)) many catecholaminergic neurons (gray) in the A1/C1 area of the VLM. Modified from (Ritter et al., 1998).
(F) Local glucoprivation in the VLM by nanoinjection of 5-thioglucose (5-TG, dashed line) completely inhibits BAT SNA and reduces BAT thermogenesis (TBAT) and metabolic oxygen consumption (EXP CO2). Modified from (Madden, 2012).
Although some locus coeruleus (LC) neurons are synaptically connected to BAT, LC neurons are not activated by cold exposure (Cano et al., 2003), nor are they required for cold-evoked BAT thermogenesis (Almeida et al., 2004). However, central PGE2-evoked thermogenesis was markedly reduced in LC-lesioned rats (Almeida et al., 2004). Inhibition of the KATP channel-dependent activation of neurons in the LC reduced BAT UCP1 mRNA expression and markedly attenuated the activation of BAT SNA following icv glucose (Tovar et al., 2013). Reintroduction of NPY expression in the ARC neurons of NPY−/− mice reduced BAT UCP1 and TBAT, as well as tyrosine hydroxylase (TH) expression in PVH and LC neurons, potentially due to NPY Y1 receptor activation in PVH (Shi et al., 2013). Although a causal relationship between the ARC NPY-induced reduction in LC neuron activity and the reduced BAT thermogenesis remains to be investigated, these results are, overall, consistent with an excitatory influence of LC neuronal activity on non-cold-defensive BAT energy expenditure.
The caudal PAG contains neurons that are multisynaptically-connected to BAT (Cano et al., 2003), presumably including those that project directly to the raphe. Excitation of caudal PAG neurons increases BAT temperature, although this excitation does not play a role in the skin cooling-evoked stimulation of BAT thermogenesis (Nakamura and Morrison, 2007). The rostral ventromedial PAG contains BAT sympathoinhibitory neurons, capable of reversing the BAT thermogenesis evoked by PGE2 into the POA or by disinhibition DMH/DA neurons (Rathner and Morrison, 2006).
An uncharacterized population of neurons in the mid-pons is responsible for a tonically-active (in anesthetized preparations) inhibition of BAT thermogenesis since transections of the neuraxis near the pontomedullary junction, but not those between the pons and DMH/DA, produce large increases in TBAT and TCORE (Amini-Sereshki and Zarrindast, 1984). Inactivation of neurons near the pontine retrorubral field produced a similar stimulation of BAT thermogenesis. Although the effect of these manipulations on BAT thermogenesis is considerable, neither the exact location of the neurons mediating this inhibition nor the physiological basis for its control has been determined.
SPINAL SYMPATHETIC MECHANISMS INFLUENCING BAT THERMOGENESIS
The discharge of BAT SPNs that determines the level of BAT SNA and BAT thermogenesis, as well as the rhythmic bursting characteristic of BAT SNA, is governed by their supraspinal and segmental inputs as well as those to the network of spinal interneurons that influence BAT SPN excitability. Serotonin in the IML activates BAT SNA and BAT thermogenesis and potentiates the BAT SNA response to NMDA injections into the IML (Madden and Morrison, 2006), potentially via 5-HT1A receptors on GABAergic interneurons in the IML and 5-HT7 receptors on BAT SPNs (Madden and Morrison, 2006). Thus, the BAT stimulating effect of 5-HT uptake inhibitors could arise from increased serotonin at spinal synapses controlling BAT activity. Spinal glutamate and 5-HT receptors play critical roles in mediating the descending excitation of BAT SPNs by their antecedent premotor neurons in the rRPa (Madden and Morrison, 2006, 2010; Nakamura et al., 2004) (Fig. 1) and mice lacking central serotonergic neurons show blunted BAT thermogenesis during cold exposure (Hodges et al., 2008). The dense dopamine beta hydroxylase innervation of BAT SPNs (Cano et al., 2003) suggests that spinal catecholamine release could modulate the activity of BAT SPNs. Activation of MC-4R on BAT sympathetic preganglionic neurons may contribute to increased BAT energy expenditure (Rossi et al., 2011). Intrathecal PACAP increases BAT SNA, presumably by increasing the activity of BAT SPNs.
DIET AND FEEDING EFFECTS ON BAT THERMOGENESIS
Food consumption and dietary composition influence the level of BAT thermogenesis, which, in addition to the heat released during digestive processes and nutrient transport, contributes to the “acute thermic effect” of food or “postprandial thermogenesis”. BAT norepinephrine turnover, an indirect index of BAT SNA, is increased following a meal. The thermic effect of food (Schwartz et al., 1988) and the feeding-evoked increase in metabolic rate are attenuated by sympatholytic drugs. The mechanisms underlying postprandial BAT thermogenesis remain unknown. Glucose administration increased β-adrenergic receptor-mediated thermogenesis (Acheson et al., 1983) and glucose and insulin administration increased BAT SNA (Holt and York, 1989; Rahmouni et al., 2004) and streptozotocin-induced diabetic rats with low insulin have impaired diet-induced BAT thermogenesis. However, there is no correlation between the insulin response to a meal and the thermic effect of feeding. Nutrient sensing in the gut, perhaps via cholecystokinin (CCK)A receptor activation of vagal afferents, may contribute to postprandial thermogenesis in BAT: lipid administration into the duodenum increases TBAT, an effect that is blocked by systemic administration of a CCKA receptor antagonist, by infusion of a local anesthetic into the duodenum, or by blockade of NMDA receptors in the NTS (Blouet and Schwartz, 2012). Interestingly, CCK can also act centrally to increase BAT SNA, although the physiological relevance is unknown. Elevations in intestinal osmolality also elicit a sympathetically-mediated BAT thermogenesis (Kobayashi et al., 2001).
In addition to the acute effects of a meal on BAT thermogenesis, the specific composition of the diet, particularly the fat content, can have chronic effects on BAT thermogenesis, i.e., diet-induced thermogenesis (Stock, 1989). Diet-induced obesity increases indirect indices (e.g., UCP-1 mRNA and UCP-1 protein expression) of BAT activation (Fromme and Klingenspor, 2011) and TBAT was elevated in obese mice on a high fat diet for 20 weeks (Enriori et al., 2011). Indeed, acute changes in diet increase NE turnover in BAT (Levin et al., 1983; Young et al., 1982) and β-adrenergic receptor signaling is required for the activation of BAT during maintenance on a high fat diet (Bachman et al., 2002). However, the initially elevated BAT NE turnover rate in high fat fed rats begins fall by 5 weeks and by 3 months is equivalent to that in rats on a control diet (Levin et al., 1983). Furthermore, at 22 days on diet, BAT SNA was lower in rats consuming a high fat compared to a low fat diet. Nonetheless, treatments that impair BAT thermogenesis, such as ablation of BAT or deletion of UCP-1 or β-adrenergic receptors, render animals prone to excess weight gain during maintenance on high fat diet (Feldmann et al., 2009; Hamann et al., 1996).
High fat or high energy diets that recruit BAT have a protein-diluting effect that may contribute to their ability to activate BAT (Cannon and Nedergaard, 2004; Stock, 1999), since low protein diets or those deficient in indispensable amino acids increase BAT activity (increased BAT UCP-1 mRNA or GDP binding) (Rothwell et al., 1983; Zhu et al., 2012) and lead to anorexia (Zhu et al., 2012), an effect that requires the anterior piriform cortex (APC). Direct detection of the amino acid deficiency by APC neurons drives the anorexia, but the role of APC neurons in the BAT activation in response to protein-deficient diets is unknown. Elevated levels of ketone bodies resulting from ketogenic diets with low carbohydrate, high protein and high fat can increase BAT SNA and energy expenditure (Srivastava et al., 2013) and decrease body weight. β-hydroxybutyrate also increases indices of BAT activity and NE turnover in BAT (Kolanowski et al., 1994). Although little is known about the underlying mechanisms, injection of β-hydroxybutyrate into the VMH or the PVH increases BAT SNA (Sakaguchi et al., 1988a).
Fasting or food restriction inhibits thermogenesis, at least in part by reducing BAT activity (Rothwell and Stock, 1982; Sivitz et al., 1999). The fasting-evoked decrease in BAT UCP-1 mRNA expression, but not the decrease in BAT UCP-1 protein level was reversed by leptin administration (Sivitz et al., 1999). Although these results might suggest that low leptin levels promote the inhibition of BAT during fasting, such data highlight the fact that isolated measures of UCP-1 mRNA are inadequate for assessing the physiologically-relevant activity state of BAT (Nedergaard and Cannon, 2013).
Both hypoglycemia and its cellular glucopenic simulation by administration of the glycolytic inhibitor, 2-deoxy-D-glucose (2-DG), cause hypothermia, at least in part by inhibiting BAT thermogenesis (Egawa et al., 1989; Madden, 2012), which occurs principally by a centrally-mediated inhibition of BAT SNA (Madden, 2012). Glucoprivic sensitivity in the lateral hypothalamus modestly reduces BAT SNA, while glucoprivation in the VLM completely inhibits BAT SNA (Madden, 2012) (Fig. 5F). Whether other brain regions, such as the VMH, implicated in glucose sensation, can contribute to the hypoglycemic inhibition of BAT SNA and BAT thermogenesis remains unknown. Regarding the neural pathways through which neurons in the VLM may mediate the glucoprivic inhibition of BAT SNA, the NPY/catecholaminergic projection from the VLM to the PVH may play an important role. Systemic glucoprivation did not attenuate BAT SNA following pontomedullary transection (Madden, 2012), consistent with a requirement for a supramedullary structure in the hypoglycemia-evoked inhibition of BAT SNA. Further, activation of PVH neurons inhibits BAT SNA (Madden and Morrison, 2009) and the NPY/catecholaminergic input from the VLM to the PVH is important for other counterregulatory responses to hypoglycemia. Additionally, a GABAA receptor-mediated inhibition of BAT sympathetic premotor neurons in the rRPa may be involved (Madden, 2012).
CONCLUSION
BAT thermogenesis is regulated primarily by a core thermoregulatory neural network (Fig. 1) which responds to skin thermoreceptor afferent signaling and to falls in core temperature to alter the sympathetic outflow to BAT. In addition to cold defense, BAT activation and elevations in body temperature, accomplished by non-thermal activation of the thermoregulatory network, occurs in a variety of behavioral states, including immunologic responses, wakefulness and stress. The high metabolic rate required for BAT thermogenesis demands a dependable supply of metabolic fuels, particularly oxygen, lipolytic by-products and glucose, and thus the neural network controlling BAT activation can be strongly influenced by permissive synaptic and hormonal signals reflecting the short- and long-term availability of the essential fuels to sustain BAT metabolism. These modulatory influences (Fig. 1) on the BAT thermoregulatory network indicate not only the complexity of the central control of this highly metabolic organ, but also the many central mechanisms determining BAT sympathetic outflow that remain to be explored. Of particular interest is the regulation of BAT activity involving the microcircuitry within the POA, the hormonal signaling to ARC neurons, the activation of the orexin neurons in the PeF/LH, and the modulation of BAT inhibitory influences mediated through the PVH and brainstem inputs to BAT sympathetic premotor neurons in the rRPa. Additionally, vagal afferents provide a spectrum of viscerosensory metabolic signals to integrative networks in the NTS that can elicit a potent modulation of BAT thermogenesis. Although there is little evidence for a central regulation of BAT thermogenesis and its attendant energy consumption that is specifically directed toward body weight regulation, it is not surprising that a reduction in BAT energy expenditure can be a predisposing factor in weight gain. Conversely, it is logical that augmented BAT activity could contribute to a reduction in body adipose stores. Further research into the functional organization of the central neural networks regulating BAT thermogenesis will not only increase our understanding of the factors controlling this metabolic furnace, but also reveal novel interventional approaches to modulating the level of BAT energy expenditure.
ACKNOWLEDGEMENTS
Support of the authors’ research that contributed to this review came from National Institutes of Health grants R01NS40987 (SFM), R01DK57838 (SFM), R56DK082558 (CJM), American Heart Association Scientist Development Grant and Grant-in-Aid (CJM), Collins Medical Trust Research Grant (DT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acheson K, Jequier E, Wahren J. Influence of beta-adrenergic blockade on glucose-induced thermogenesis in man. J. Clin. Invest. 1983;72:981–986. doi: 10.1172/JCI111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Coimbra NC, Branco LG. Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J. Physiol. 2004;558:283–294. doi: 10.1113/jphysiol.2004.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Sereshki L, Zarrindast MR. Brain stem tonic inhibition of thermoregulation in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984;247:R154–159. doi: 10.1152/ajpregu.1984.247.1.R154. [DOI] [PubMed] [Google Scholar]
- Arruda AP, Milanski M, Romanatto T, Solon C, Coope A, Alberici LC, Festuccia WT, Hirabara SM, Ropelle E, Curi R, Carvalheira JB, Vercesi AE, Velloso LA. Hypothalamic actions of tumor necrosis factor alpha provide the thermogenic core for the wastage syndrome in cachexia. Endocrinology. 2010;151:683–694. doi: 10.1210/en.2009-0865. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Bajzer M, Olivieri M, Haas MK, Pfluger PT, Magrisso IJ, Foster MT, Tschop MH, Krawczewski-Carhuatanta KA, Cota D, Obici S. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia. 2011;54:3121–3131. doi: 10.1007/s00125-011-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;276:R1569–1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem. Cell Biol. 2005;123:147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS ONE. 2012;7:e51898. doi: 10.1371/journal.pone.0051898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratincsak A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience. 2004;127:385–397. doi: 10.1016/j.neuroscience.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Valdivia S, Reynaldo M, Cyr NE, Nillni EA, Perello M. Short-term cold exposure activates TRH neurons exclusively in the hypothalamic paraventricular nucleus and raphe pallidus. Neurosci. Lett. 2012;518:86–91. doi: 10.1016/j.neulet.2012.04.059. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R277–290. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Cardinal P, Bellocchio L, Clark S, Cannich A, Klugmann M, Lutz B, Marsicano G, Cota D. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology. 2012;153:4136–4143. doi: 10.1210/en.2012-1405. [DOI] [PubMed] [Google Scholar]
- Cerri M, Mastrotto M, Tupone D, Martelli D, Luppi M, Perez E, Zamboni G, Amici R. The inhibition of neurons in the central nervous pathways for thermoregulatory cold defense induces a suspended animation state in the rat. J Neurosci. 2013;33:2984–2993. doi: 10.1523/JNEUROSCI.3596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–638. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Cerri M, Morrison SF. Corticotropin releasing factor increases in brown adipose tissue thermogenesis and heart rate through dorsomedial hypothalamus and medullary raphe pallidus. Neuroscience. 2006;140:711–721. doi: 10.1016/j.neuroscience.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia ML, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Role of corticotrophin-releasing factor in effects of leptin on sympathetic nerve activity and arterial pressure. Hypertension. 2001;38:384–388. doi: 10.1161/01.hyp.38.3.384. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J. Neurosci. 2013;33:4825–4833. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Fontes MA, DiMicco JA. Cardiovascular and thermal responses evoked from the periaqueductal grey require neuronal activity in the hypothalamus. J. Physiol. 2009;587:1201–1215. doi: 10.1113/jphysiol.2008.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov EL, Kim YY, Usdin TB. Regulation of hypothalamic signaling by tuberoinfundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation. J. Neurosci. 2011;31:18166–18179. doi: 10.1523/JNEUROSCI.2619-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA. Lateral hypothalamic injection of 2-deoxy-D-glucose suppresses sympathetic activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1989;257:R1386–1392. doi: 10.1152/ajpregu.1989.257.6.R1386. [DOI] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA. Preoptic area injection of corticotropin-releasing hormone stimulates sympathetic activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1990;259:R799–806. doi: 10.1152/ajpregu.1990.259.4.R799. [DOI] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991;260:R328–334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Fernandes-Santos C, Zhang Z, Morgan DA, Guo DF, Russo AF, Rahmouni K. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology. 2013;154:2481–2488. doi: 10.1210/en.2012-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R1–8. doi: 10.1152/ajpregu.00411.2010. [DOI] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Griffiths EC, Rothwell NJ, Stock MJ. Thermogenic effects of thyrotrophin-releasing hormone and its analogues in the rat. Experientia. 1988;44:40–42. doi: 10.1007/BF01960238. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010;12:431–442. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ. Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Deng C, Huang XF. The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain. CNS Drugs. 2013;27:423–434. doi: 10.1007/s40263-013-0062-1. [DOI] [PubMed] [Google Scholar]
- Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H197–206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SJ, York DA. Interaction of intracerebroventricular insulin and glucose in the regulation of the activity of sympathetic efferent nerves to brown adipose tissue in lean and obese Zucker rats. Brain Res. 1989;500:384–388. doi: 10.1016/0006-8993(89)90336-3. [DOI] [PubMed] [Google Scholar]
- Jung KM, Clapper JR, Fu J, D'Agostino G, Guijarro A, Thongkham D, Avanesian A, Astarita G, DiPatrizio NV, Frontini A, Cinti S, Diano S, Piomelli D. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012;15:299–310. doi: 10.1016/j.cmet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Bruning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat. Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T, Inoue S, Kimura S. Thermogenesis induced by intravenous infusion of hypertonic solutions in the rat. J. Physiol. 2001;535:601–610. doi: 10.1111/j.1469-7793.2001.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski J, Young JB, Landsberg L. Stimulatory influence of D(−)3-hydroxybutyrate feeding on sympathetic nervous system activity in the rat. Metabolism. 1994;43:180–185. doi: 10.1016/0026-0495(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kirigiti M, Lindsley SR, Loche A, Madden CJ, Morrison SF, Smith MS, Grove KL. Efferent projections of neuropeptide Y-expressing neurons of the dorsomedial hypothalamus in chronic hyperphagic models. J. Comp. Neurol. 2013;521:1891–1914. doi: 10.1002/cne.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Triscari J, Sullivan AC. Altered sympathetic activity during development of diet-induced obesity in rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1983;244:R347–355. doi: 10.1152/ajpregu.1983.244.3.R347. [DOI] [PubMed] [Google Scholar]
- Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, Ananthakrishnan G, Rohner-Jeanrenaud F, Drucker DJ, DiMarchi R, Rahmouni K, Oldfield BJ, Tschop MH, Perez-Tilve D. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012;61:2753–2762. doi: 10.2337/db11-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martinez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Dieguez C, Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J. Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R224–232. doi: 10.1152/ajpregu.00449.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R320–325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J. Physiol. 2005;566:559–573. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J. Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R831–843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R776–783. doi: 10.1152/ajpregu.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Tupone D, Cano G, Morrison SF. alpha2 Adrenergic receptor-mediated inhibition of thermogenesis. J. Neurosci. 2013;33:2017–2028. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R832–837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front. Endocrinol. (Lausanne) 2012;3 doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, Pittelli M, Cavone L, Pugliese AM, Moroni F, Chiarugi A. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J. Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R127–136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat. Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc. Natl. Acad. Sci. USA. 2010;107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur. J. Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim. Biophys. Acta. 2013;1831:943–949. doi: 10.1016/j.bbalip.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Niijima A, Rohner-Jeanrenaud F, Jeanrenaud B. Role of ventromedial hypothalamus on sympathetic efferents of brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984;247:R650–654. doi: 10.1152/ajpregu.1984.247.4.R650. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J. Appl. Physiol. 2011;110:789–798. doi: 10.1152/japplphysiol.00128.2010. [DOI] [PubMed] [Google Scholar]
- Osaka T. Heat loss responses and blockade of prostaglandin E2-induced thermogenesis elicited by alpha1-adrenergic activation in the rostromedial preoptic area. Neuroscience. 2009;162:1420–1428. doi: 10.1016/j.neuroscience.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, Ahima RS. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes. 2006;55:3091–3098. doi: 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981;289:401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Moghadam KK, Maggi LS, Donadio V, Vetrugno R, Liguori R, Zoccoli G, Poli F, Pizza F, Pagotto U, Ferri R. Autonomic disturbances in narcolepsy. Sleep Med. Rev. 2011;15:187–196. doi: 10.1016/j.smrv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ, Fekete C, Latorre R, Nanni C, Bucci M, Clemens LE, Heldmaier G, Watanabe M, Leste-Lassere T, Maitre M, Tedesco L, Fanelli F, Reuss S, Klaus S, Srivastava RK, Monory K, Valerio A, Grandis A, De Giorgio R, Pasquali R, Nisoli E, Cota D, Lutz B, Marsicano G, Pagotto U. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J. Clin. Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: a source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077:99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res. 1998;805:41–54. doi: 10.1016/s0006-8993(98)00655-6. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Barnes MJ, Hermann GE. Leptin “gates” thermogenic action of thyrotropin-releasing hormone in the hindbrain. Brain Res. 2009;1295:135–141. doi: 10.1016/j.brainres.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. A role for insulin in the diet-induced thermogenesis of cafeteria-fed rats. Metabolism. 1981;30:673–678. doi: 10.1016/0026-0495(81)90082-2. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Effect of chronic food restriction on energy balance, thermogenic capacity, and brown-adipose-tissue activity in the rat. Biosci. Rep. 1982;2:543–549. doi: 10.1007/BF01314214. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ, Tyzbir RS. Mechanisms of thermogenesis induced by low protein diets. Metabolism. 1983;32:257–261. doi: 10.1016/0026-0495(83)90190-7. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Arase K, Bray GA. Effect of intrahypothalamic hydroxybutyrate on sympathetic firing rate. Metabolism. 1988a;37:732–735. doi: 10.1016/0026-0495(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Takahashi M, Bray GA. Diurnal changes in sympathetic activity. Relation to food intake and to insulin injected into the ventromedial or suprachiasmatic nucleus. J. Clin. Invest. 1988b;82:282–286. doi: 10.1172/JCI113584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, Holmberg KH, Klein I, Klaus J, Gomez LF, Kolb H, Secrest J, Jochems J, Myashiro K, Buckley P, Hadcock JR, Eberwine J, Conti B, Bartfai T. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59:43–50. doi: 10.2337/db09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, DiMicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur. J. Neurosci. 2007;26:2228–2238. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Jaeger LF, Veith RC. Effect of clonidine on the thermic effect of feeding in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988;254:R90–94. doi: 10.1152/ajpregu.1988.254.1.R90. [DOI] [PubMed] [Google Scholar]
- Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, Enriquez RF, Baldock PA, Zhang L, Sainsbury A, Herzog H, Lin S. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Fink BD, Donohoue PA. Fasting and leptin modulate adipose and muscle uncoupling protein: divergent effects between messenger ribonucleic acid and protein expression. Endocrinology. 1999;140:1511–1519. doi: 10.1210/endo.140.4.6668. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R417–428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Baxa U, Niu G, Chen X, Veech RL. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life. 2013;65:58–66. doi: 10.1002/iub.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc. Natl. Acad. Sci. USA. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Molchanova AY, Dogan MD, Patel S, Petervari E, Balasko M, Wanner SP, Eales J, Oliveira DL, Gavva NR, Almeida MC, Szekely M, Romanovsky AA. The hypothermic response to bacterial lipopolysaccharide critically depends on brain CB1, but not CB2 or TRPV1, receptors. J. Physiol. 2011;589:2415–2431. doi: 10.1113/jphysiol.2010.202465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J. Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock MJ. The role of brown adipose tissue in diet-induced thermogenesis. Proc. Nutr. Soc. 1989;48:189–196. doi: 10.1079/pns19890029. [DOI] [PubMed] [Google Scholar]
- Stock MJ. Gluttony and thermogenesis revisited. Int. J. Obes. Relat. Metab. Disord. 1999;23:1105–1117. doi: 10.1038/sj.ijo.0801108. [DOI] [PubMed] [Google Scholar]
- Szekely M. The vagus nerve in thermoregulation and energy metabolism. Auton. Neurosci. 2000;85:26–38. doi: 10.1016/S1566-0702(00)00217-4. [DOI] [PubMed] [Google Scholar]
- Tabarean IV. Persistent histamine excitation of glutamatergic preoptic neurons. PLoS ONE. 2012;7:e47700. doi: 10.1371/journal.pone.0047700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Zhang W, Sameshima K, Kuroki C, Matsumoto A, Sunanaga J, Kono Y, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for prostaglandin E2-induced fever and defence against environmental cooling in mice. J Physiol. 2013 doi: 10.1113/jphysiol.2013.261271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar S, Paeger L, Hess S, Morgan, Donald A, Hausen AC, Brönneke, Hella S, Hampel B, Ackermann PJ, Evers N, Büning H, Wunderlich FT, Rahmouni K, Kloppenburg P, Brüning, Jens C. KATP-Channel-Dependent Regulation of Catecholaminergic Neurons Controls BAT Sympathetic Nerve Activity and Energy Homeostasis. Cell Metab. 2013;18:445–455. doi: 10.1016/j.cmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, Oka Y, Katagiri H. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab. 2012;16:825–832. doi: 10.1016/j.cmet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF. Central Activation of the A1 Adenosine Receptor (A1AR) Induces a Hypothermic, Torpor-Like State in the Rat. J. Neurosci. 2013;33:14512–14525. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1049–1058. doi: 10.1152/ajpregu.00640.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty AN, Allen AM, Oldfield BJ. The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology. 2010;151:4236–4246. doi: 10.1210/en.2009-1235. [DOI] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R992–1002. doi: 10.1152/ajpregu.00516.2006. [DOI] [PubMed] [Google Scholar]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, Lopez M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144:4692–4697. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, Olson DP, Tong Q. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE. 2012;7:e45167. doi: 10.1371/journal.pone.0045167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Gandhi N, Lai M, Kublaoui BM. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS ONE. 2012;7:e36453. doi: 10.1371/journal.pone.0036453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Masaki T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125:535–540. doi: 10.1016/j.neuroscience.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J. Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, Hubschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur. J. Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- Young JB, Saville E, Rothwell NJ, Stock MJ, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J. Clin. Invest. 1982;69:1061–1071. doi: 10.1172/JCI110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABAA receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R110–116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 2011a;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Morgan DA, Kuburas A, Thedens DR, Russo AF, Rahmouni K. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes. 2011b;60:1063–1071. doi: 10.2337/db10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Krasnow SM, Roth-Carter QR, Levasseur PR, Braun TP, Grossberg AJ, Marks DL. Hypothalamic signaling in anorexia induced by indispensable amino acid deficiency. Am. J. Physiol. Endocrinol. Metab. 2012;303:E1446–1458. doi: 10.1152/ajpendo.00427.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]