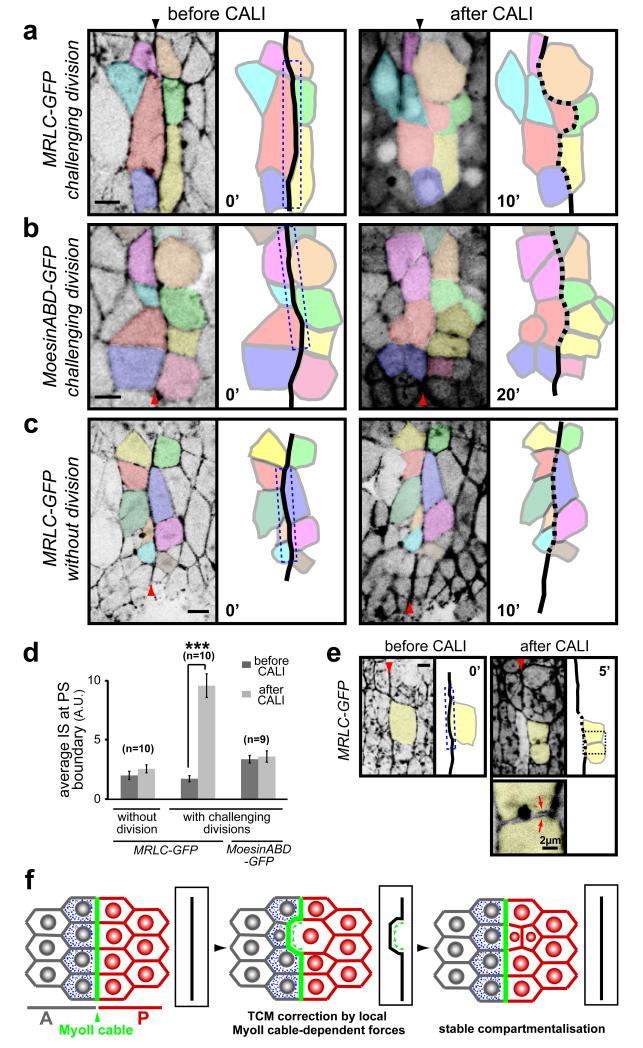
Fig. 5. CALI inactivation of the MyoII cable gives cell sorting defects at PS boundaries.
(a-c) Movie frames showing CALI performed on the PS boundary (dashed box) in embryos expressing MRLC-GFP (a, c) or Moesin-ABD-GFP (b). (a) In presence of the division of an anterior boundary cell (coloured in red), CALI inactivation of MyoII at the cable leads to an irregular PS boundary (dashed). After division, one daughter cell invades the posterior compartment. (b) A similar experiment in Moesin-ABD-GFP control embryos does not affect cell sorting at the boundary, even after four of the boundary cells have divided. (c) CALI targeting of the MyoII cable in MRLC-GFP embryos in absence of divisions: no cell sorting defects are observed in those conditions. (d) Quantification of membrane straightness after CALI at PS boundaries, in presence and absence of boundary cell divisions. Data are expressed as mean ± SEM. The symbol * indicates a statistical comparison between conditions before and after CALI in the indicated contexts, using paired Student’s t test and with *** corresponding to p<0.001. (e) Cable inactivation (dashed box) does not affect the MyoII pool required for division of the targeted boundary cell (yellow), since the cytokinesis ring forms and the cell divides normally (panel 5′). Arrows on the close-up view show MyoII localising at the cortex of the newly formed membranes within each daughter cells. (f) Model of cell sorting at Drosophila embryonic lineage restriction boundaries. TCM: transient cell mixing.