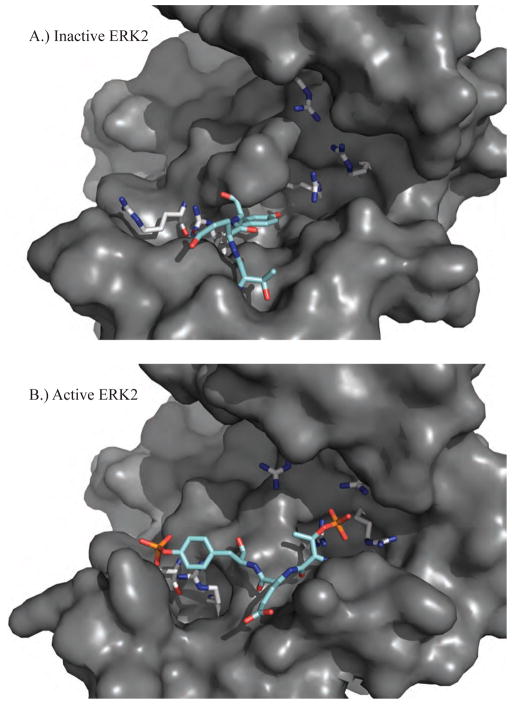
Figure 3.
A.) The inactive structure of ERK2 (PDB ID: 1ERK) shows the unphosphorylated T-X-Y motif (cyan) located on the activation loop (L12) of protein kinases (183T-E-Y185 for ERK1/2). B.) The active structure of ERK2 (PDB ID: 2ERK) has been phosphorylated on both the threonine and tyrosine residues that favor conformational rearrangements stabilized by electrostatic interactions between the negatively charged phosphorylated residues and positively charged arginine residues (grey).