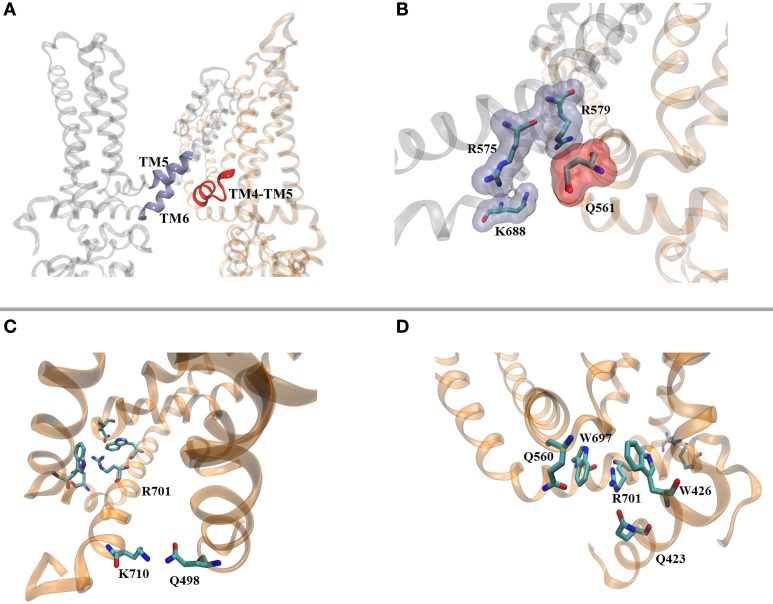
Figure 2.
PIP2 mediates intra- and inter-subunit contacts. (A) Identified PIP2 binding pocket in the structure of TRPV1. (B) Residues proposed as mediators of PIP2 interaction to the channel. (C) K710 is located at the distal end of the TD helix and interacts with Q498 located at the bottom of TM2. (D) Predicted/potential cation-π interactions connecting the TRP box of the TD helix with the TM4-TM5 helix and N-terminal region. In order to get a better placement of the lateral chains of the available structure, the TRPV1 channel (PDB ID 3J5P) was embedded in lipids (POPC), and placed in a periodic box containing water and ions (140 mM KCl). After 5 ns of all-atom MD simulations using the NAMD/CHARMM32 force-field, the channel was visualized using VMD.