Figure 1. Hypothetical model of α-syn toxicity and spread of pathology in PD and PDD.
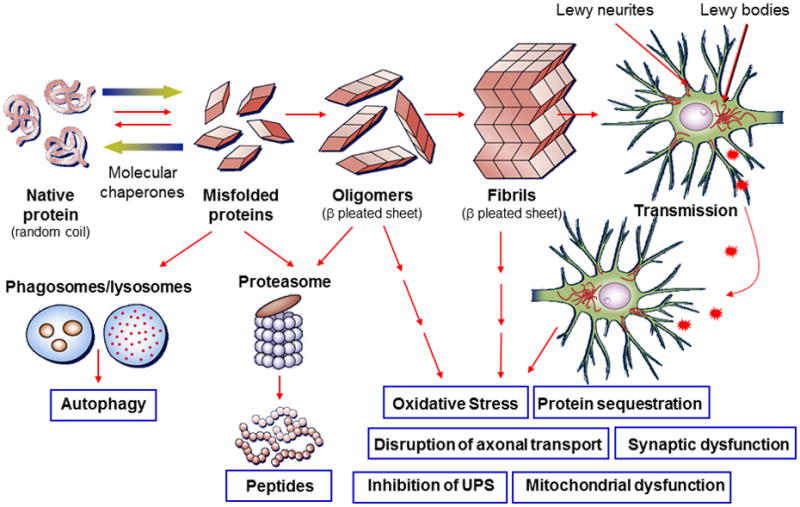
From left to right in the top row: native α-syn in normal conditions exists in a soluble random coil state and under pathological conditions undergoes misfolding into pathogenic species of α-syn (dimers, trimers, oligomers, etc.) that further aggregate into higher order structures (protofibrils, other intermediates and fibrils) which ultimately are the building blocks for pathological inclusions visualized under light microscopy at autopsy (i.e. Lewy bodies and Lewy Neurites).66 Genetic abnormalities and environmental factors may accelerate this process.27-29, 66, 119, 121 Normal quality-control systems (chaperones, ubiquitin proteosome and phagosome–lysosome systems) that prevent or reverse protein misfolding or eliminate misfolded proteins are overwhelmed (indicated by dashed lines).66 Remarkably, recent data suggest that the progression of PD and related disorders may be linked to the cell-to-cell spread of pathological species of α-syn,57-59, 64 as illustrated in the upper right of the figure. The inter-related toxic consequences of pathological α-syn transmission are listed in the lower right of the figure.66 It is unclear which species of pathogenic α-syn is directly toxic to neurons; however recent animal studies show that synthetic α-syn fibrils alone are sufficient to transmit disease, i.e. α-syn pathology, between neurons and cause clinical disease (indicated by the furthest right arrow).58, 59
