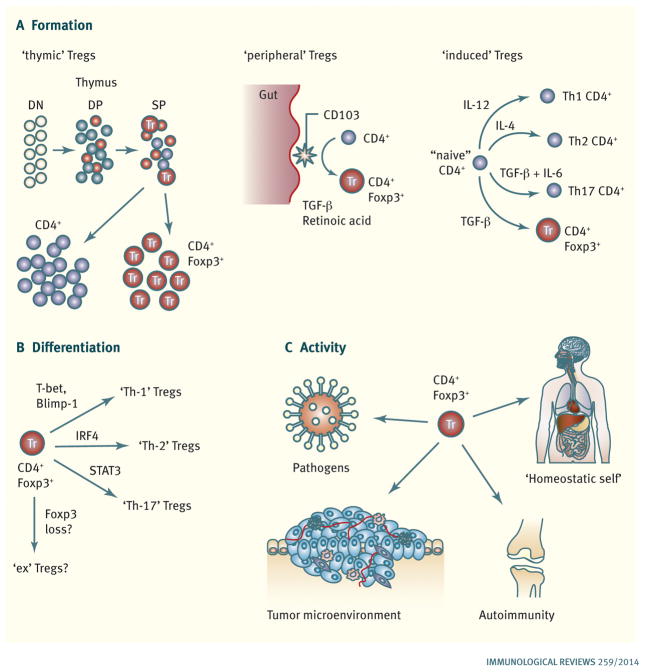
Fig. 1. Major themes in regulatory T cell biology.
(A). Pathways leading to Treg cell formation. CD4+Foxp3+ ‘thymic’ Treg cells are a major source and are generated during T-cell development in the thymus. Conventional CD4+ T cells can undergo conversion to become CD4+Foxp3+ ‘peripheral’ Treg cells in the periphery, most notably in response to CD103+ dendritic cells presenting microbial and food antigens encountered via the gut (by a TGF-β- and retinoic acid-dependent mechanism). Conventional CD4+ T cells can also become ‘induced’ Foxp+ Treg cells in response to TCR stimulation in the presence of TGF-β in vitro by a process that is analogous to the cytokine-driven differentiation of conventional CD4+ effector T cells into different effector subsets (Th-1, Th-2, and Th-17 cells). (B). Treg cell differentiation. Following activation, Foxp3+ Treg cells can use the same transcription factors required for the acquisition of effector phenotypes by conventional CD4+ T cells to adopt novel characteristics that allow them to suppress CD4+ T cells with the corresponding phenotype. There is also evidence that Treg cells can also lose Foxp3 expression and become ‘ex-Tregs’ that could potentially possess autoaggressive properties. (C). In vivo Treg cell activity. Foxp3+ Treg cells are required to prevent the development of an autoagressive lymphoproliferative disease and maintain quiescence in the steady state immune system (‘homeostatic self’). Foxp3+ Treg cells have also been implicated in and/or shown to be able to modulate ongoing immune responses in settings such and infection, autoimmunity, and cancer.