Figure 6.
Functional Consequences of the OTULIN-LUBAC Interaction
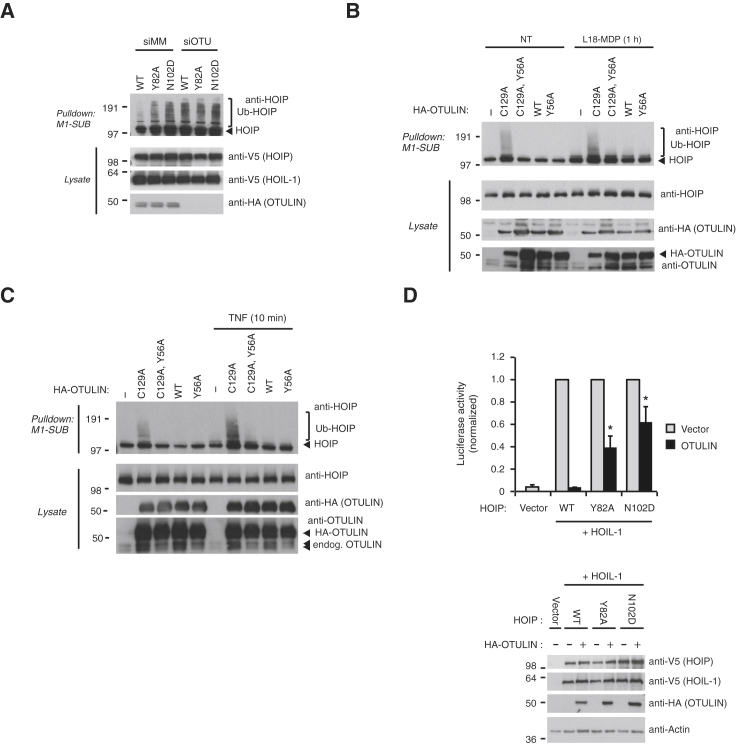
(A) Purification of endogenous Ub conjugates with Met1-specific Ub binding domain (Keusekotten et al., 2013) in lysates of HEK293T control and OTULIN-depleted cells transfected with HOIP variants and HOIL-1. Purified material and lysate was examined by immunoblotting. Mutation of the HOIP PIM pocket results in spontaneous accumulation of Met1-linked polyubiquitin on HOIP.
(B and C) Purification of endogenous Ub conjugates with M1-SUB in U2OS and NOD2 cells transfected with the indicated OTULIN variants and treated with L18-MDP (B) or TNF (C). Purified material was analyzed as in (A). Mutation of the OTULIN PIM impairs stabilization of HOIP ubiquitination by catalytic inactive (C129A) OTULIN under basal conditions and after stimulation.
(D) NFκB reporter activity in lysates of HEK293T cells transfected with HOIL-1, HOIP, or HOIP PIM pocket mutants and with or without the expression of OTULIN. OTULIN abrogated NFκB activity induced by wild-type LUBAC but was less effective in inhibiting activity induced by HOIP PIM pocket mutants.