Figure 1.
BCLAF1 Interacts with Phosphorylated BRCA1 following DNA Damage
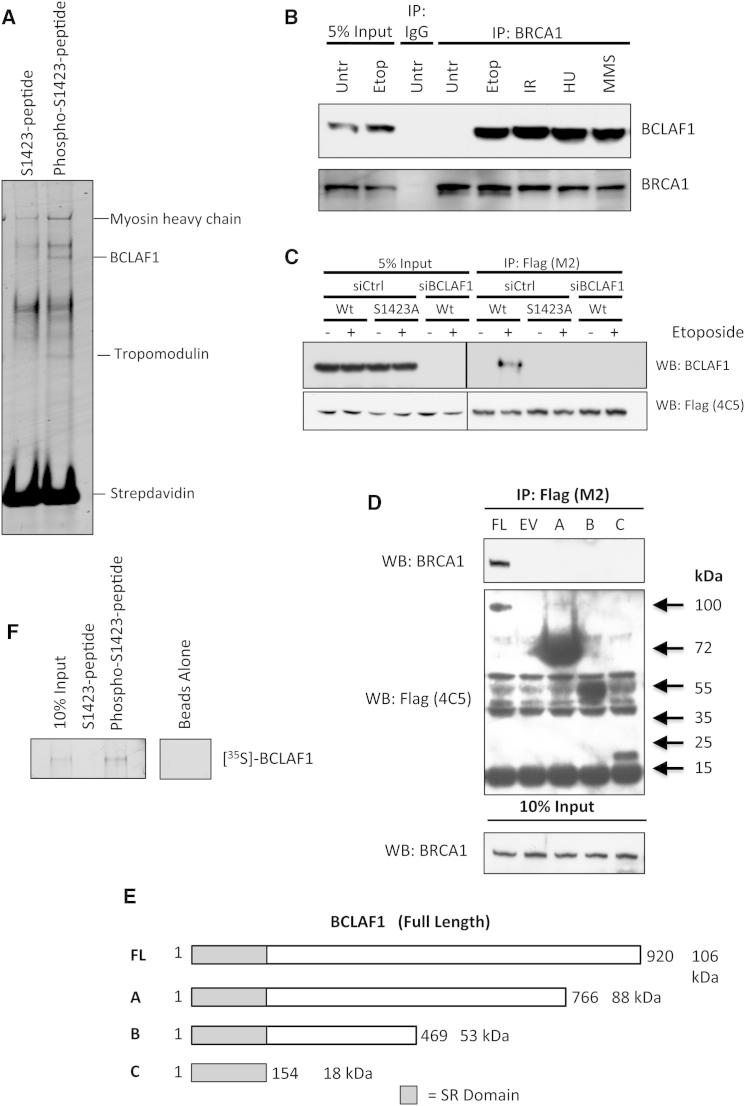
(A) Colloidal Coomassie-stained gel of peptide pull-down assays carried out from 293T cell nuclear extracts with phosphorylated BRCA1-S1423 peptide and its nonphosphorylated counterpart. The indicated phosphopeptide-interacting band was identified as BCLAF1 by LC-MS/MS.
(B) Coimmunoprecipitation assay demonstrating an interaction between BRCA1 and BCLAF1 in 293T cells treated with etoposide (1 μM, 16 hr), IR (2 Gy, 1 hr), MMS (200 μM, 6 hr), and HU (5 μM, 3 hr).
(C) Coimmunoprecipitation assay demonstrating DNA damage-induced interaction of BCLAF1 with ectopic Flag-BRCA1 is abrogated by BRCA1-S1423A phosphosite substitution. A Flag-BRCA1 IP was also carried out from cells depleted of BCLAF1 to confirm the specificity of the BCLAF1 antibody.
(D) Mapping of the BRCA1-interacting region within BCLAF1. Coimmunoprecipitation experiments were carried out from etoposide-treated cells transfected with the Flag-BCLAF1 truncation mutant constructs depicted in (E).
(E) Schematic diagram of BCLAF1 truncation constructs used for BRCA1 coimmunoprecipitation experiments in (D).
(F) Peptide pull-down assays carried out with [35S] in vitro-translated BCLAF1, indicating that BCLAF1 interacts directly and specifically with the phosphorylated BRCA1-S1423 peptide and not its unphosphorylated counterpart. See also Figure S1.