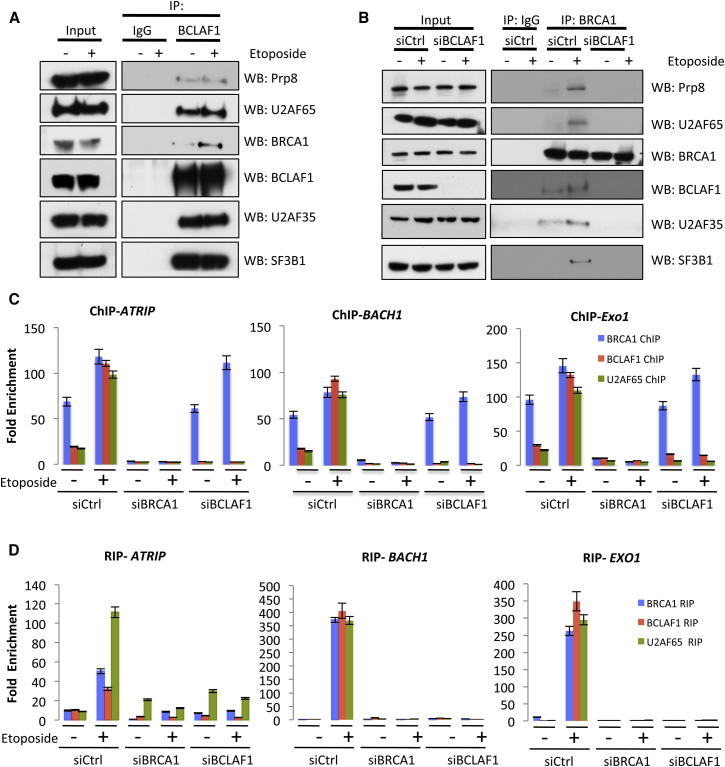
Figure 3.
BRCA1/BCLAF1 Forms an mRNA Splicing Complex which Is Recruited to Target Gene Promoters and Transcripts following DNA Damage
(A) Coimmunoprecipitation assays demonstrating that BCLAF1 interacts with the spliceosome proteins Prp8, U2AF65, U2AF35, and SF3B1 in both the presence and absence of DNA damage.
(B) Coimmunoprecipitation assays demonstrating DNA damage-induced interaction between BRCA1 and the spliceosome proteins Prp8, U2AF65 U2AF35, and SF3B1 in response to DNA damage. Additionally, depletion of BCLAF1 results in abrogation of DNA damage-induced interaction between BRCA1 and these proteins.
(C) BRCA1, BCLAF1, and U2AF65 ChIP-qPCRs demonstrating constitutive binding of BRCA1 to ATRIP, BACH1, and EXO1 promoters irrespective of DNA damage in control (siCtrl) cells. The ChIPs also demonstrate that BCLAF1 and U2AF65 are recruited to these promoters only in etoposide-treated cells and that depletion of BRCA1 or BCLAF1 results in loss of DNA damage-induced BCLAF1 and U2AF65 recruitment, respectively. Graphs represent the mean fold enrichment quantified from three independent experiments ± SEM.
(D) BRCA1, BCLAF1, and U2AF65 RIP-qRT-PCRs demonstrating that BRCA1, BCLAF1, and U2AF65 only bind to ATRIP, BACH1, and EXO1 mRNAs in response to DNA damage. In addition, depletion of BCLAF1 results in loss of BRCA1 and U2AF65 mRNA binding to all three transcripts. Graphs represent the mean fold enrichment quantified from three independent experiments ± SEM. See also Figure S3.