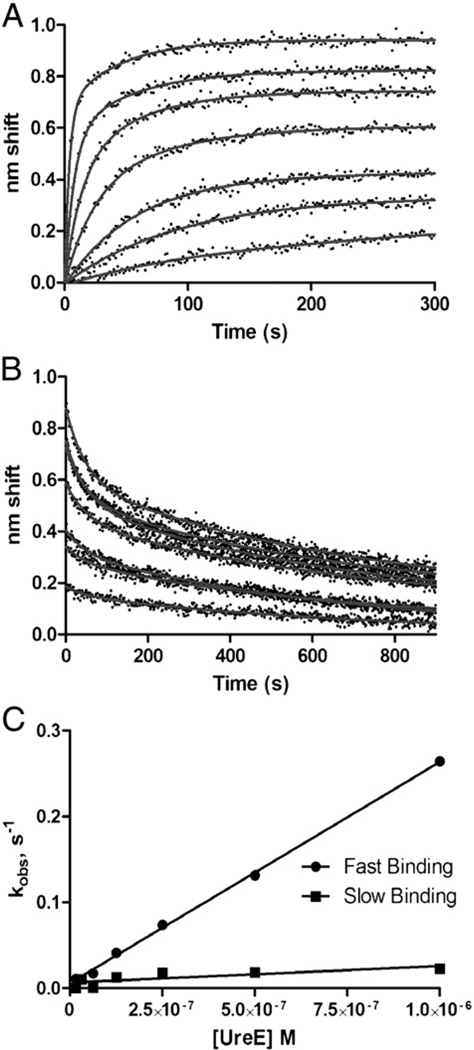
Fig. 2.
Kinetic analysis of HypA–UreE binding by BLI. A. Association phase. Sensor-immobilized, biotinylated HypA was exposed to (from bottom to top) 16, 32, 63, 125, 250, 500 or 1000 nM analyte UreE for 300 s. B. Dissociation phase. Immediately after association, sensors were moved to buffer only and dissociation was observed for 300 s. Both association and dissociation were fit to parallel two-state models. Off rate constants were determined to be 0.02 s−1 and 1.4×10−3 s−1. All data are reference-subtracted and nonspecific binding was negligible (data not shown). C. Determination of on rate constants. Observed rate constants determined from fits of the association phase plotted against analyte concentration yield slopes (= kon) of 2.6×105, M−1 s−1 for the fast state and 1.9×104 M−1 s−1 for the slow state.