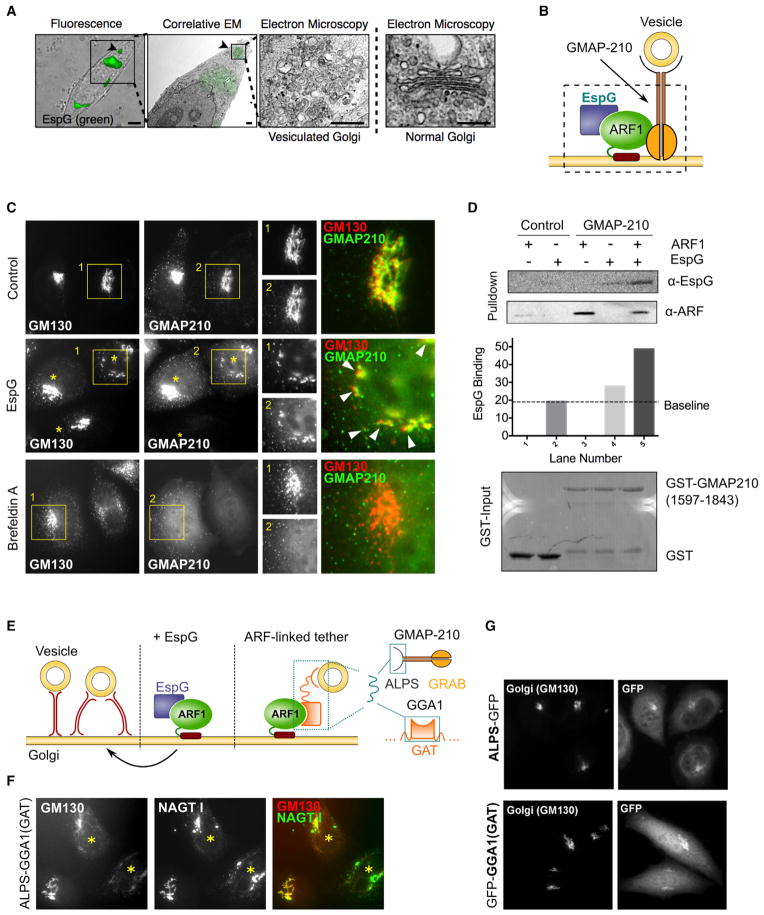
Figure 6. Disruption of the Golgi Architecture through ARF1-Dependent Membrane Tethering.
(A) CLEM shows the vesicular ultrastructure of the Golgi after microinjection with EspG, which remains constrained to areas positive for EspG signal.
(B) Identification of golgin GMAP-210 as an ARF1-dependent membrane tether involved in the EspG mechanism of Golgi disassembly by linking vesicles to membranes and preventing their transport.
(C) Fluorescent micrographs showing the distribution of GMAP-210 relative to the Golgi (GM130). GMAP-210 is retained on fragmented membranes following disassembly of the Golgi by EspG (arrowheads), in contrast to when ARF1 is removed from the membranes by BFA treatment.
(D) Pull-down experiment showing the binding of EspG to GMAP-210/ARF1 complex.
(E) Cartoon schematic illustrating the design of an ARF1-dependent, membrane-tethering chimera using a vesicle-binding motif linked to an ARF1-GTP-binding domain of GGA1. Our model of EspG function predicts vesicle-to-membrane linkage that is driven by the presence of GTP-ARF1 on membranes.
(F) Overexpression of the ARF1-dependent, membrane-tethering chimera is sufficient to disrupt the Golgi architecture. Transfected cells are marked with an asterisk.
(G) Control experiments show no impact on Golgi organization due to overexpression of either a vesicle-binding motif or an ARF1-GTP-binding domain alone in the absence of a direct tether.
See also Figure S5.